-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
check this out https://en.wikipedia.org/wiki/Lophophine , any chance that it could cause hallucinations?About as active as MDPEA I guess.
Solipsis
Bluelight Crew
- Joined
- Mar 12, 2007
- Messages
- 15,509
That methoxy on the bottom left is out of place for it to be an analogue of lophophine, it would have to be on the bottom carbon of the right-side ring. On what position of a naphthyl you put substitutions can matter quite a bit for its toxicity in the human body, although I don't recall a methoxy being one of the more worrisome - but that position is tricky for some other substitutions.
Because the methoxy is out of place instead of lophophine-like it^ is like MDPEA with a methoxy sticking out of the methylenedioxy bridge (well in that vicinity) - and that is a position known to be very sensitive for benzodioxole style empathogens - messing with it usually makes it inactive.
So not a good chance for any activity, no - first better to consistently choose what drug yours will be an analogue of by having something sort of bioisosteric group - like MDMA vs. PAL-287 and then avoid the issues the parent drugs are known to have.
Also worth mentioning that PAL-287 but it seems also the analogue of that with a methoxy on the same position your suggested compound has it, are found to be MAO-A inhibitors quite a bit more potent than amphetamine. But that's the alpha-methyl version which yours doesn't have - which makes it more sensitive to MAO degradation (and I bet less inhibiting of MAO), and the activity would have to compensate for that - MDPEA needs very high doses or MAOI / is hardly active at all and lophophine is also not potent but is at least a direct MMDA analogue, being basically MMDPEA.
Finally - I'm getting bad flashes of PMA but that's not actually related.
Because the methoxy is out of place instead of lophophine-like it^ is like MDPEA with a methoxy sticking out of the methylenedioxy bridge (well in that vicinity) - and that is a position known to be very sensitive for benzodioxole style empathogens - messing with it usually makes it inactive.
So not a good chance for any activity, no - first better to consistently choose what drug yours will be an analogue of by having something sort of bioisosteric group - like MDMA vs. PAL-287 and then avoid the issues the parent drugs are known to have.
Also worth mentioning that PAL-287 but it seems also the analogue of that with a methoxy on the same position your suggested compound has it, are found to be MAO-A inhibitors quite a bit more potent than amphetamine. But that's the alpha-methyl version which yours doesn't have - which makes it more sensitive to MAO degradation (and I bet less inhibiting of MAO), and the activity would have to compensate for that - MDPEA needs very high doses or MAOI / is hardly active at all and lophophine is also not potent but is at least a direct MMDA analogue, being basically MMDPEA.
Finally - I'm getting bad flashes of PMA but that's not actually related.
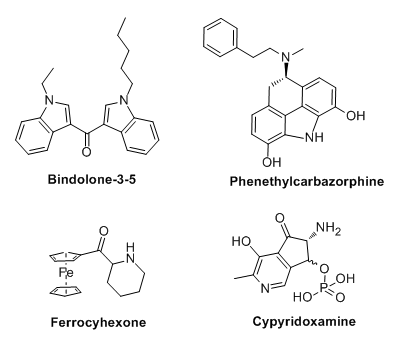
1. Cannabimimetic bisindole; shoud satisfy the requirement for binding, one side is pentyl going to hydrophobic tail pocket,
another tail is propyl, similar length to 4-ethyl, or 4-methoxy in naphthalene case which enhance activity.
2. Carbazole-like structure instead of morphinan, with correct stereoisomer pointing the amine, which phenethyl attached for more activity. It also looks similar to tricyclic TCA, antimuscarinics and antihistamines.
3. Just for fun of more 3-D structure construction, May be inactive or toxic via metal residue.
4. Another molecule based on cyclized pyridoxine (vit B6) making it cathinone-like. just fun to imagine but I supposed this is also inactive due to polarity of phosphate ester.
P.S. Compound 1 is active in vivo; anecdotally
Jonneh
Bluelighter
- Joined
- Jul 29, 2014
- Messages
- 226
I've thought of this.
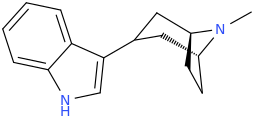
You want the nitrogen to be a primary amine. The ethyl spacing is taken up by the tropane ring.
I like this. I wonder how much of the tropane ring will be perceived as primary amine bulk (in which case it might behave like a DET-EPT/aeruginascin hybrid) versus the analogue of alpha-carbon substitution (alpha,N-DMT, anyone?). This will also apply to my 4-HO-MiPT azetidine analogue from the last page - is that 4-methyl part of the isopropyl analogue or rather an alpha-substitution equivalent?:
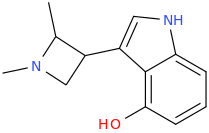
Last edited:
SKL
Bluelight Crew
- Joined
- Sep 15, 2007
- Messages
- 14,647
I think I'd like this:
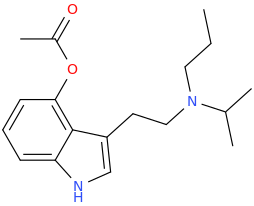
Was 4-AcO/HO-PiPT never made when PiPT was around?
I think not. But would welcome correction. As I said elsewhere, I'd expect it to be less potent but at a relevant dose at least as psychedelic and perhaps longer lasting than 4-AcO/HO-MiPT; but that's complete conjecture.
Google reveals one Bluelight post mentioning 5-MeO-EiPT circulating ca. 2013 and at least one reddit user claiming to have tried 5-MeO-PiPT but that's about the closest thing I could find … not very close … I liked 5-MeO-MiPT a bunch but the longer ones seemed less interesting, something about this structure though seems promising to me …
Last edited:
Solipsis
Bluelight Crew
- Joined
- Mar 12, 2007
- Messages
- 15,509
I think it's already a bit of a bother to make an asymmetrical trypt, but apparently 4-HO-DPT is a pain to make as well IIRC... so 4-AcO-PiPT or something like that is probably a lot of work.
Yeah I like the azetidine too! Thought of something like that a while back, but rather a more straightforward version (4-hydroxy optional):
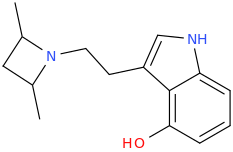
The methyl antlers are also optional of course, depending on whether you want to make an analogue of DMT, MET or DET ... but if you were to analyze or assay different isomers it is probably interesting to add both methyls and see which one is specifically active. Possibly it is less strict than with LSZ... apparently on tryptamines much more freedom is allowed than on lysergamides.
What is a slight concern though, is that you would move in the direction of say this one:
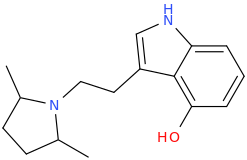
which bears close resemblance to Pyr-T which Shulgin found to be a horrible substance. Possibly due to its metabolism? IDK
I would also be quite curious about freaky analogues like these (haha the one on the right looks funny - and a real bitch to get opsin to draw wedges since there aren't actually chiral centers):
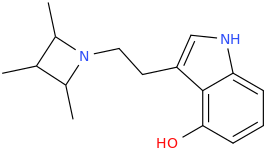
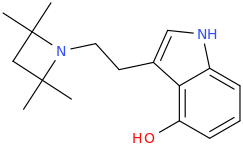
Yeah I like the azetidine too! Thought of something like that a while back, but rather a more straightforward version (4-hydroxy optional):

The methyl antlers are also optional of course, depending on whether you want to make an analogue of DMT, MET or DET ... but if you were to analyze or assay different isomers it is probably interesting to add both methyls and see which one is specifically active. Possibly it is less strict than with LSZ... apparently on tryptamines much more freedom is allowed than on lysergamides.
What is a slight concern though, is that you would move in the direction of say this one:

which bears close resemblance to Pyr-T which Shulgin found to be a horrible substance. Possibly due to its metabolism? IDK
I would also be quite curious about freaky analogues like these (haha the one on the right looks funny - and a real bitch to get opsin to draw wedges since there aren't actually chiral centers):
NSFW:


Last edited:
aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
I think it's already a bit of a bother to make an asymmetrical trypt, but apparently 4-HO-DPT is a pain to make as well IIRC... so 4-AcO-PiPT or something like that is probably a lot of work.
Yeah I like the azetidine too! Thought of something like that a while back, but rather a more straightforward version (4-hydroxy optional):

The methyl antlers are also optional of course, depending on whether you want to make an analogue of DMT, MET or DET ... but if you were to analyze or assay different isomers it is probably interesting to add both methyls and see which one is specifically active. Possibly it is less strict than with LSZ... apparently on tryptamines much more freedom is allowed than on lysergamides.
What is a slight concern though, is that you would move in the direction of say this one:

which bears close resemblance to Pyr-T which Shulgin found to be a horrible substance. Possibly due to its metabolism? IDK
I would also be quite curious about freaky analogues like these (haha the one on the right looks funny - and a real bitch to get opsin to draw wedges since there aren't actually chiral centers):
NSFW:


The methyl groups in the first compound do not bind in the same hydrophobic pockets as the methyl groups in LSZ. Instead, the methyl groups here bind in the same place as the methyl group on the aliphatic nitrogen in LSD. In LSD/LSZ, the alkyl groups which have a significant effect on potency are the ones connected to the non-basic amide nitrogen, which binds in a different (and weaker) way than the charged aliphatic nitrogen.
Solipsis
Bluelight Crew
- Joined
- Mar 12, 2007
- Messages
- 15,509
I know: I said apparently on tryptamines much more freedom is allowed than on lysergamides.
Maybe with 2,4-dimethylazetidine you won't see huge differences between isomers but I bet that at some point if the alkyl chains get longer the conformation becomes a problem and there would be countless ways to constrain the antlers together using bridges / rings which would still tell us something about whether DPT analogues work better when the antlers are tied together or spread apart, or bent. So comparing spatial conformations would still be interesting a la LSZ even if there is much less of a small particularly shaped pocket?
Again: the metabolism might be a concern, or another reason why Pyr-T was so bad...
Maybe with 2,4-dimethylazetidine you won't see huge differences between isomers but I bet that at some point if the alkyl chains get longer the conformation becomes a problem and there would be countless ways to constrain the antlers together using bridges / rings which would still tell us something about whether DPT analogues work better when the antlers are tied together or spread apart, or bent. So comparing spatial conformations would still be interesting a la LSZ even if there is much less of a small particularly shaped pocket?
Again: the metabolism might be a concern, or another reason why Pyr-T was so bad...
Jonneh
Bluelighter
- Joined
- Jul 29, 2014
- Messages
- 226
Yeah I like the azetidine too! Thought of something like that a while back, but rather a more straightforward version (4-hydroxy optional):

What is a slight concern though, is that you would move in the direction of say this one:

which bears close resemblance to Pyr-T which Shulgin found to be a horrible substance. Possibly due to its metabolism? IDK
Yeah, I had Pyr-T in mind when deciding where to put the azetidine (there needed to be one somewhere, obviously!). I reasoned that not having inflexible bulk hanging off the very end might help avoid going in the Pyr-T direction. Come to think of it, all the di-somethings beyond DMT have something physically iffy going on, or else are freaky in another respect (tremors with DET, strange vestibular effects of DiPT and audio trickery with DiPT and DPT). Fusing the antlers to form Pyr-T doesn't go well, which is what made me scared of going where you went with the azetidine (fused isopropyls).
As you say, though, it could be a (fairly specific) metabolic issue and thus might not apply to the azetidine. And mine might turn out to be too stubby. Oh, and fortune favours the brave.
Last edited:
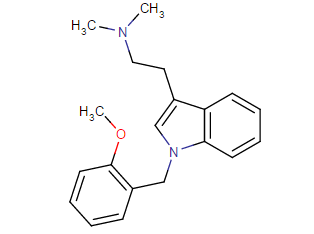
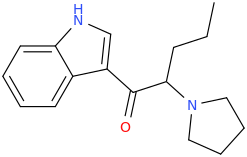
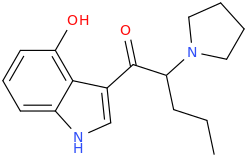
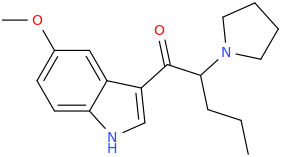
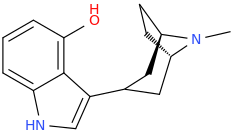
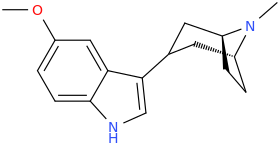
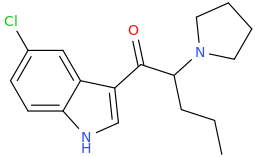
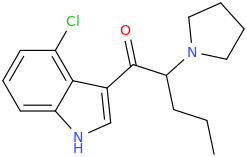
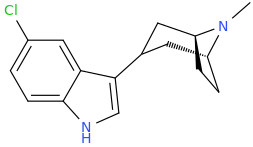
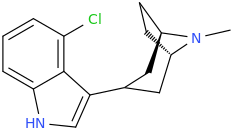
^ those indoles with a bulky cyclic tertiary amine more likeky will have strong anticholinergic like this
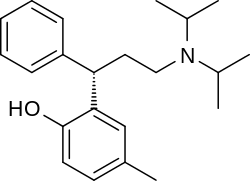
Tolterodine a central muscarinic anticholinergic causing VERY interesting hallucinations populated with zombies getting up from graves and attacking you Nasty stuff.. google: "detrol nightmares" So go easy on lipophilicity with the indoles and it should be fine..

Tolterodine a central muscarinic anticholinergic causing VERY interesting hallucinations populated with zombies getting up from graves and attacking you Nasty stuff.. google: "detrol nightmares" So go easy on lipophilicity with the indoles and it should be fine..
- Status
- Not open for further replies.