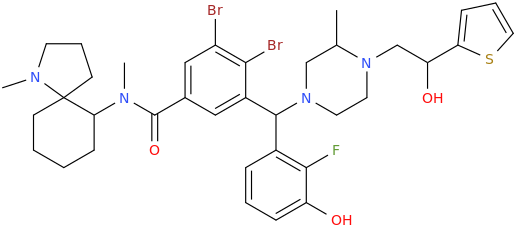
-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
GHB anhydride will react with itself giving the GHB-GHB ester dimer
Or if the solution is dilute, it will intramolecularly react with itself to give one molecule of GBL and one molecule of GHB.
Solipsis
Bluelight Crew
- Joined
- Mar 12, 2007
- Messages
- 15,509
Oh yeah, that's too bad - thought it would be nasty but at least survives... any way to make poly-GHB or something of the sort though? :D Oh yeah obviously, just a polyester - makes polybutyrate basically - a biodegradable plastic that does use 1,4-BDO in its synthesis... fun plastics - speaking of fun plastics and BDO:
spiroxetamine is a cool name, and reminds me remotely of DXM with one of the rings unhinged... but no that ^ stuff is pretty shuffled compared to that. I wonder what lies in between though... will try to draw something later..
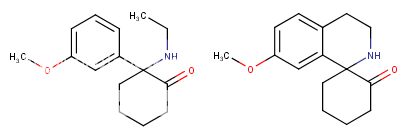
Would it still be called a spiro compound if the N-alkyl chain loops back to the cyclohexane ring instead of the phenyl ring? I guess you'd have to check out the 7 possible compounds you can make by attaching that alkyl chain to see what kind of constraining improves potency. Hooking it up to some of the right places might also prevent metabolism, although most dissociatives suffer from too long duration rather than too short.
Could a carbonyl be placed on one of the rings of DXM that would be the equivalent position of where K / MXE have it, and be expected to have decent effects on activity?
Bindeez (also marketed as Aqua Dots,[1] Beados,[2] and Pixos[3]) are a children's toy. It was subject to a multi-national product recall after it was found that the Wangqi Product Factory in Shenzhen, China had used a cheap chemical that was a pharmacologically active sedative prodrug instead of the safer specified one in some shipped toys, resulting in the illness and hospitalization of some children who ingested the beads
spiroxetamine is a cool name, and reminds me remotely of DXM with one of the rings unhinged... but no that ^ stuff is pretty shuffled compared to that. I wonder what lies in between though... will try to draw something later..
Would it still be called a spiro compound if the N-alkyl chain loops back to the cyclohexane ring instead of the phenyl ring? I guess you'd have to check out the 7 possible compounds you can make by attaching that alkyl chain to see what kind of constraining improves potency. Hooking it up to some of the right places might also prevent metabolism, although most dissociatives suffer from too long duration rather than too short.
Could a carbonyl be placed on one of the rings of DXM that would be the equivalent position of where K / MXE have it, and be expected to have decent effects on activity?
Last edited:
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
Various restricted-rotation methylphenidate analogs
These aren't mine, these actually have been tested for affinity, as per above link (my WP inclusion of the following):
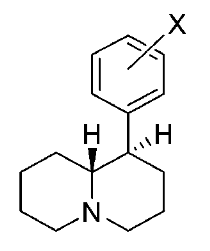
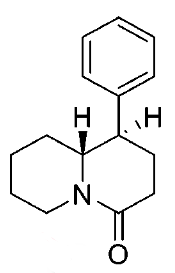
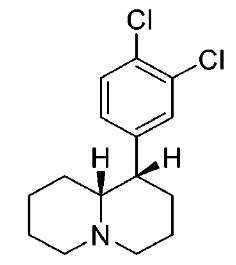
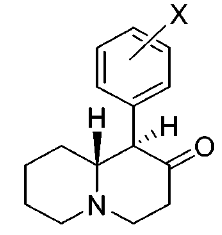
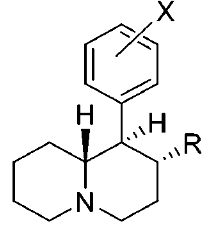
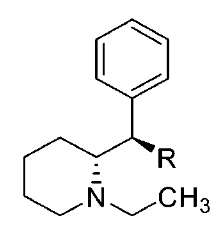
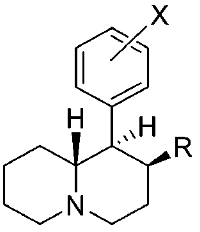
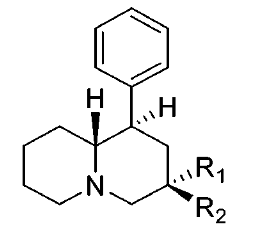
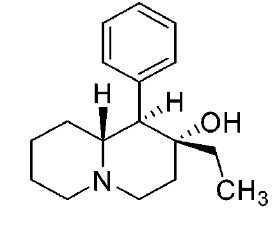
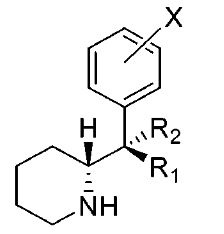
These aren't mine, these actually have been tested for affinity, as per above link (my WP inclusion of the following):










any way to make poly-GHB or something of the sort though? :D Oh yeah obviously, just a polyester - makes polybutyrate basically - a biodegradable plastic that does use 1,4-BDO in its synthesis...
That's very easy:
- Just perform a ring-opening polymerization (ROP) using either (snip!), (snip!) or (snip!) (Me-heh
- For that 1,4-BD is is used for synthesis of PBS (Polybutylene Succinate)
Our stomach cannot effectively hydrolyze them; though
There are morphinans with a keto attached like ketocyclazocineCould a carbonyl be placed on one of the rings of DXM that would be the equivalent position of where K / MXE have it, and be expected to have decent effects on activity?
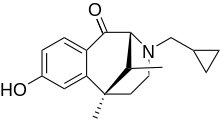
Most are Kappa selective agonists dysphoric the levo-rotatory group. But am not sure if ketocyclazocine congener of DXM ie with the "right" stereochemistry (ie dextro) are known...:
levarphanol ---> ketocylazocine (and similar morphinans)
dextromethorphan--->ketomethorphan? Oxo-methorphan??
Solipsis
Bluelight Crew
- Joined
- Mar 12, 2007
- Messages
- 15,509
To answer my own question (I think) though: I didn't have an easy time trying to figure it out, but I think it's not possible because the 9-position of DXM which seems to correspond best with where some of the ACAs have their carbonyl, is a tertiary carbon because of the phenanthrene structure.
Thats interesting though! ^
Thats interesting though! ^
adder
Bluelighter
- Joined
- Mar 28, 2006
- Messages
- 2,852
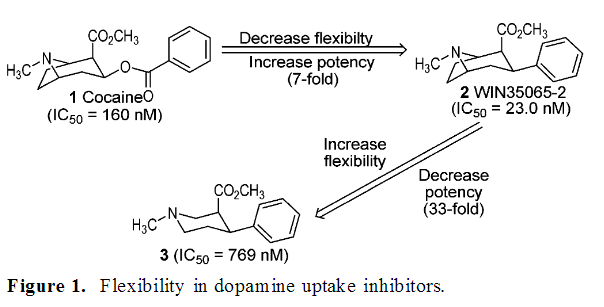
The thing with decreasing flexibility is that if a restricted molecule is able to bind in an active conformation, the activity increases, but if it can't, the activity will drop. That's why they tested different compounds in that research on methylphenidate analogues trying to put the carbonyl into different fixed spaces. By restricting molecule conformationally you basically make it less capable of unbinding via rotation around certain bonds, I guess.
adder
Bluelighter
- Joined
- Mar 28, 2006
- Messages
- 2,852
There are morphinans with a keto attached like ketocyclazocine

Most are Kappa selective agonists dysphoric the levo-rotatory group. But am not sure if ketocyclazocine congener of DXM ie with the "right" stereochemistry (ie dextro) are known...:
levarphanol ---> ketocylazocine (and similar morphinans)
dextromethorphan--->ketomethorphan? Oxo-methorphan??
10-ketodextromethorphan was tried in some research and it didn't turn out to be interesting for sure, if I remember well, it was even weaker than DXM itself as a NMDA antagonist or whatever. But anyway, the keto moiety decreases basicity of the amine, the same thing must be happening in ketamine as well, though its potency is likely decreased by o-chloro as well, yet the qualitative gain is more than potency drop. For toxicity it's probably bad though.
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
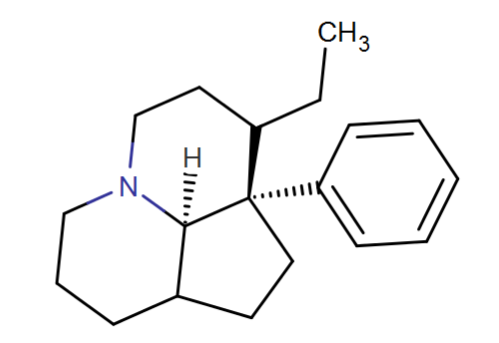
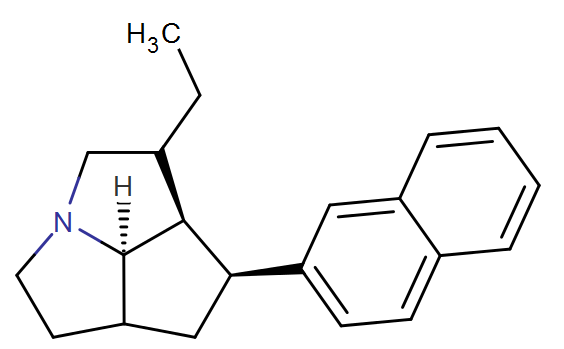
This is based on further rigidifying the quinolizidine MPH RRAs in a way that shouldn't hinder/hamper binding. I really like the look of it.
The two functionalities are facing in differing places because of the limit of bonds, when they should be R, I tweaked it a bit:
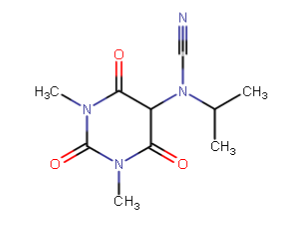
Started with caffeine and butalbital, ended up with this:
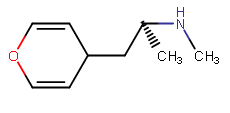
Well 'la-tee-dah' it's planar (aryl is, anyway) should work like m-amp:
What happens if you pull those labile oxygens off of the 3 & 6 positions of morphine? (I extended the six because I couldn't see that doing anything but lengthening half life) =
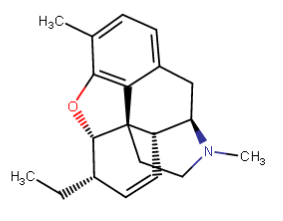
Last edited:
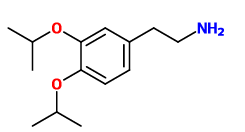
^ Im guessing this one is inactive
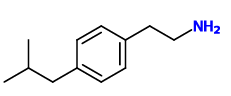
^ inspired by ibuprofen
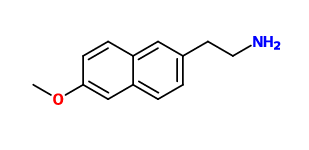
^inspired by naproxen
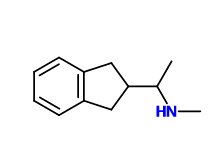
^ idk about his one
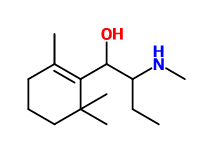
^ inspired from the guy who was obsessed about damascone

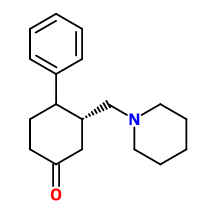
I couldn't even remember when I drew this one but it looks interesting.
btw im new to this place and I have been lurking this thread for a couple months and finally decided to share.
10-ketodextromethorphan was tried in some research and it didn't turn out to be interesting for sure, if I remember well, it was even weaker than DXM itself as a NMDA antagonist or whatever. But anyway, the keto moiety decreases basicity of the amine, the same thing must be happening in ketamine as well, though its potency is likely decreased by o-chloro as well, yet the qualitative gain is more than potency drop. For toxicity it's probably bad though.
I see..One would expect them to behave similarly to ketamine or MXE. But I think your're right: the alfa-keto group would decrease the pKa of the amine hugely(2 log units??. That's probably the reason why they suck? Note that in ketamine and PCP congener MXE, the pKa is still way higher than physiological pH (7.4). For ketamine it is about 8.5 which means much of molecule will be charged in the body anyway. So I guess in the case of K and MXE, it wont really affect binding. I am not sure what the pKa of the keto analog of DXM would be.??
NB:In the case of amphetamines and cathinones though, I always thought the drop of the pKa of the amine by the keto group has something to do with their pharmacological profile: ie from DRI (Amphetamine-like) ------> DRA (cocaine like cathinones). Cathinones pKa is much lower than that of the corresponping AMPH. At the very least, it would affect their pharmacokinetic like absorption and brain distribution.
adder
Bluelighter
- Joined
- Mar 28, 2006
- Messages
- 2,852
I don't mean the effect of pKa with respect to the ratio of charged and uncharged form in the blood. The molecules clearly get to the brain in their freebase form as a charged amine can't pass the blood-brain barrier) and the amine gets protonated at the target site interacting with an aminoacid residue with a positively charged side-chain, so the drop in basicity here could mean lower affinity at the NMDA receptor site DXM/DXO interacts with, but if it's a meaningful drop I have no idea. I imagine the carbonyl group itself is electronically and/or sterically unfavoured there for the binding, I doubt such a small change in an otherwise lipophilic molecule could affect the amount that gets to the brain much. I tried overlaying DXM with ketamine and dizocilpine some time ago and if there is any position in DXM that might correlate with the carbonyl carbon of ketamine, it is not C10, but rather C14 which is a tertiary carbon atom (I'm using the morphinan numbering here, so it's the carbon atom that bears an extra hydroxy group in oxymorphone vs. hydromorphone). However, the site that DXO binds to may not be the same site that ketamine binds to, I remember reading an article on how DXO effects were blocked by some specific ligand that didn't block ketamine or some other arylcyclohexanamine effects (or the other way around, I'd have to find it again), the conclusion was that the site for DXO is a different one. If it is so, then comparing DXO structure with ketamine and planning structural changes based on SAR of arylcyclohexanamines makes little sense anyway.
Soulfake
Bluelighter
- Joined
- Aug 12, 2010
- Messages
- 160
"7-Spiroindanyloxymorphone" - sounds and looks pretty nice, I hope a few original morphinanes will come to the rc market.
https://en.wikipedia.org/wiki/7-Spiroindanyloxymorphone
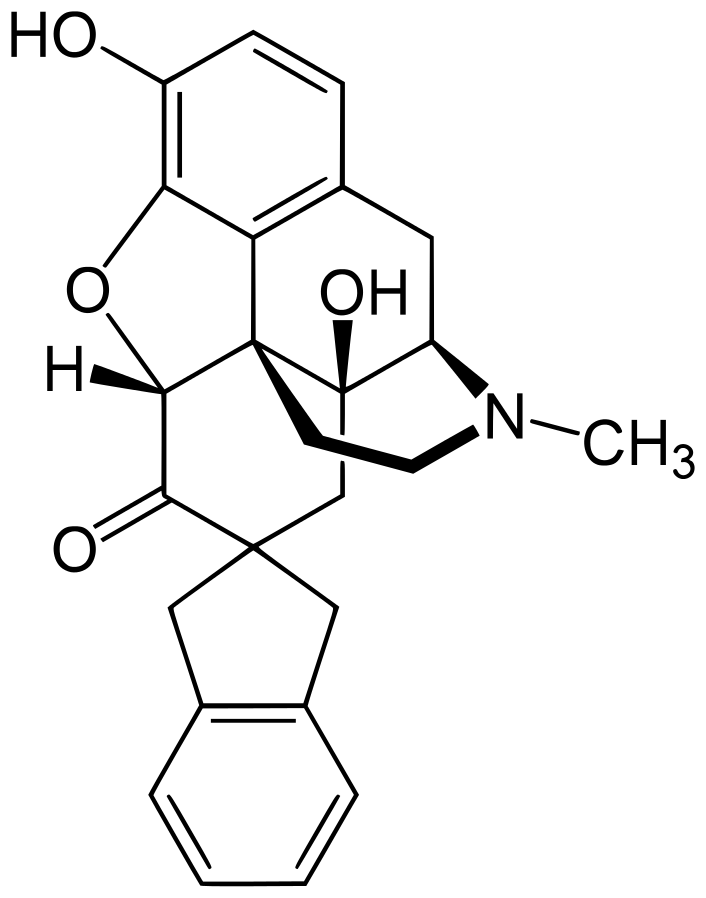
Here some things that could have nice opioid action:
https://en.wikipedia.org/wiki/7-Spiroindanyloxymorphone

Here some things that could have nice opioid action:

- Status
- Not open for further replies.