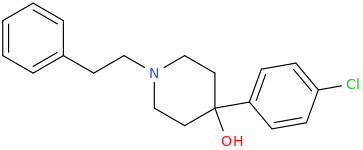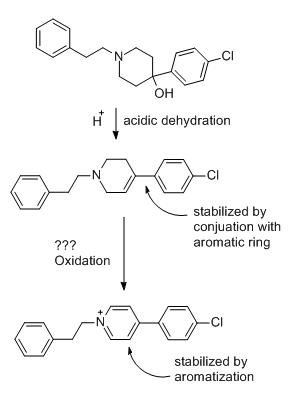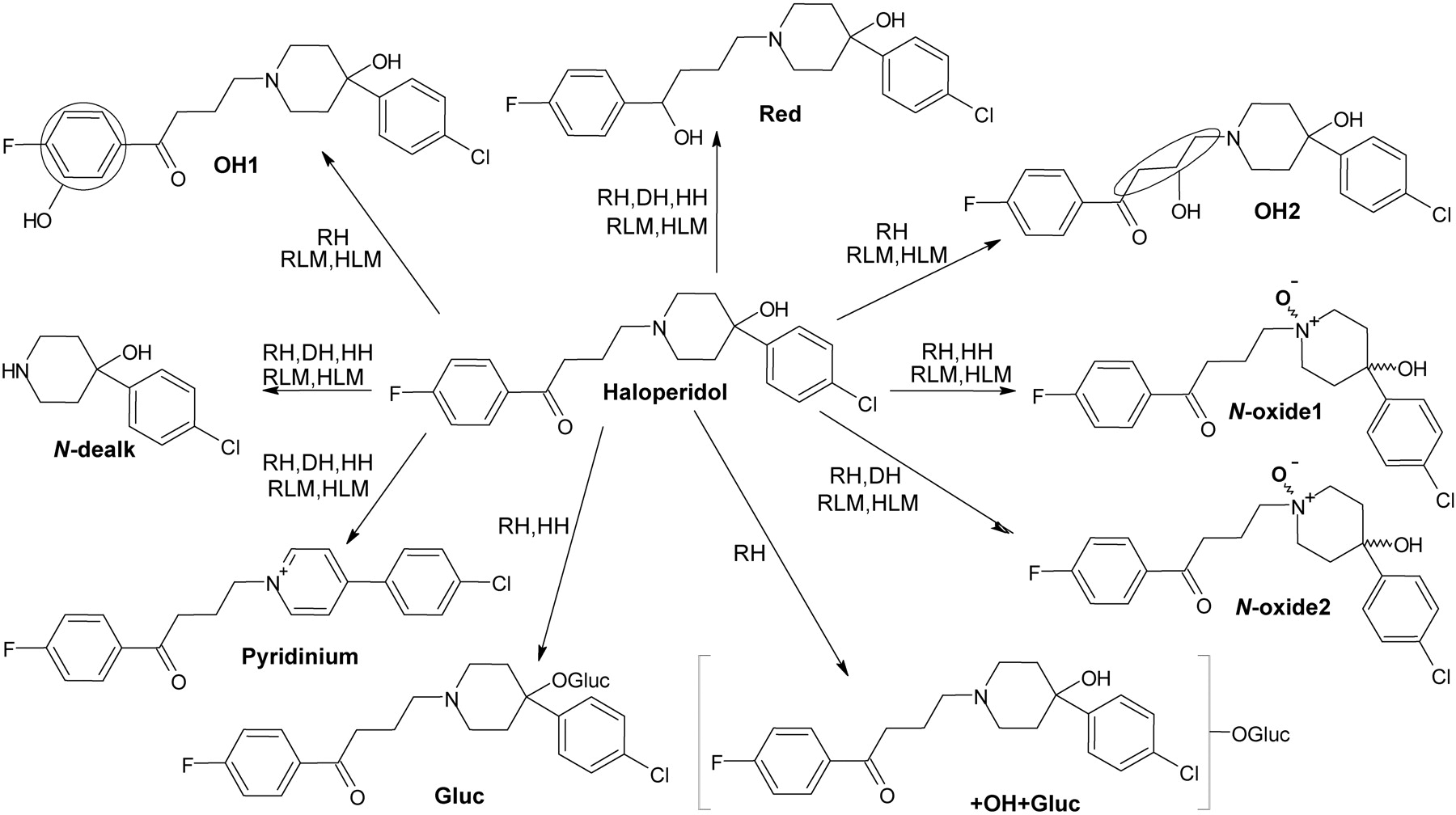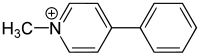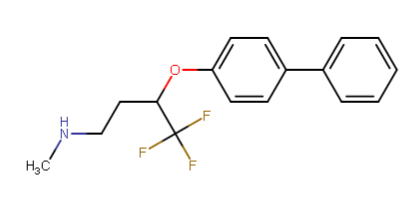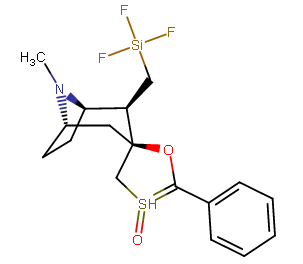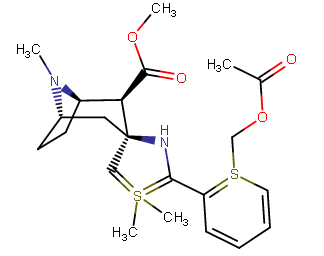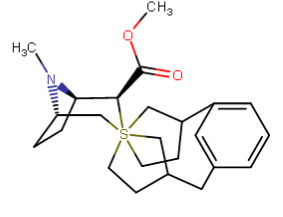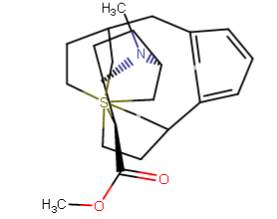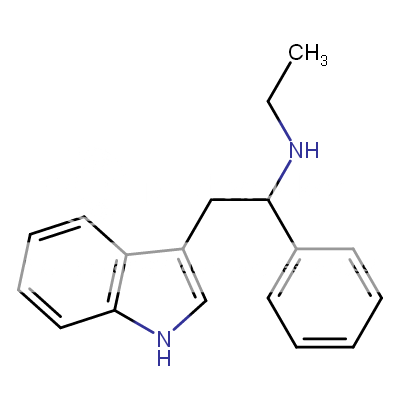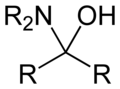
A hemiaminal (also carbinolamine) is a functional group or type of chemical compound that has a hydroxyl group and an amine attached to the same carbon atom..... Those generated from primary amines are unstable to the extent that they have never been isolated and very rarely been observed directly. In a 2007 study a hemiaminal substructure trapped in the cavity of a host-guest complex was studied with a chemical half-life of 30 minutes.... https://en.wikipedia.org/wiki/Hemiaminal