Dina Mahmoud
Greenlighter
- Joined
- Nov 6, 2014
- Messages
- 2
Hi, how are you all? Hoping to be fine
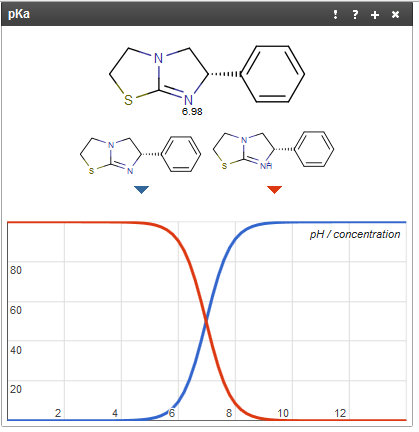
I tried to get information about pka and pkb of some chemical agents, but I don't understand how to know the nature of a certain drug, I mean Is it an acidic or basic drug? And Is it a weak or strong one? For example, Levamisole Hcl (its structure is presented in the link below); is an anthelmintic drug, in the hydrocholide salt, freely soluble in water and has pKa value of 8.
1- Is it an acidic or basic agent?
2- Is there a general rule or pKa range helps me to detect the acidic or alkaline nature of other drugs?
Hoping to help and simplify this issue to me. Thanks in advance

https://www.caymanchem.com/app/template/Product.vm/catalog/14874
I tried to get information about pka and pkb of some chemical agents, but I don't understand how to know the nature of a certain drug, I mean Is it an acidic or basic drug? And Is it a weak or strong one? For example, Levamisole Hcl (its structure is presented in the link below); is an anthelmintic drug, in the hydrocholide salt, freely soluble in water and has pKa value of 8.
1- Is it an acidic or basic agent?
2- Is there a general rule or pKa range helps me to detect the acidic or alkaline nature of other drugs?
Hoping to help and simplify this issue to me. Thanks in advance
https://www.caymanchem.com/app/template/Product.vm/catalog/14874
Last edited: