Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
...violate any rules?
R1-3: any combination of H (not a het.at., but i.e. no carbon bond) a single methylene unit (planar, beta or alpha) a double bond, or a triple bond (where it doesn't make more than four by being adjacent to a double, of course)
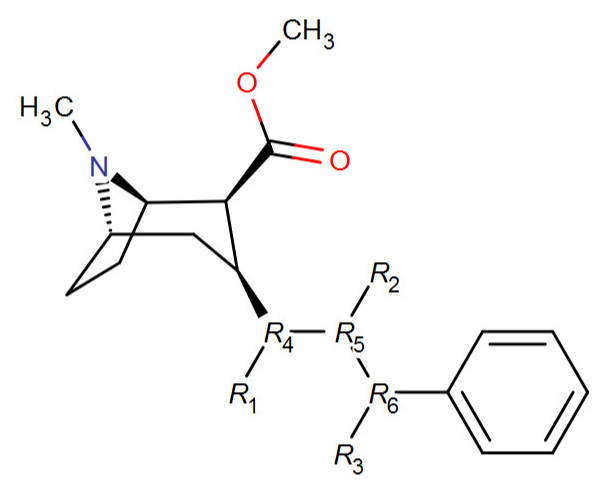
There's no oxygens, but being pure carbon (besides the number of bonds) are there any rules I am breaking with certain combinations (rules like for instance possible with a heteroatom: 'keto–enol tautomerism') of the above?
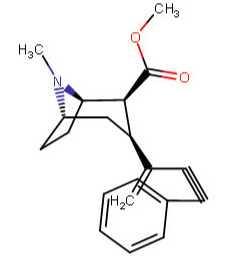
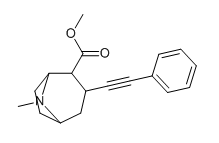
I ask because of the drastic difference between 224a and 224d with binding; with the incredible selectivity of binding versus uptake of 224c, why weren't extra-carbon bonds tried along it in differing combinations?
I'll show some individual instances besides my R-substitution image above:
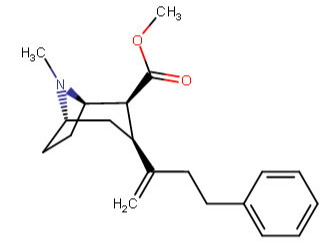
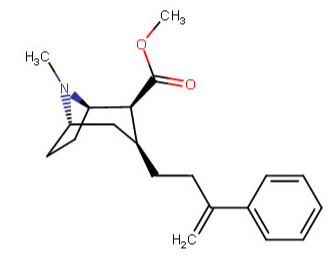
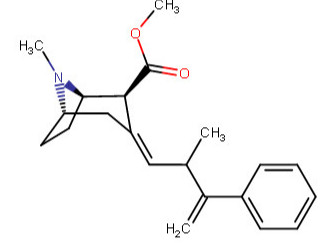
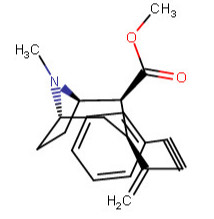
^^This one feeling like it is violating something, is it?^^
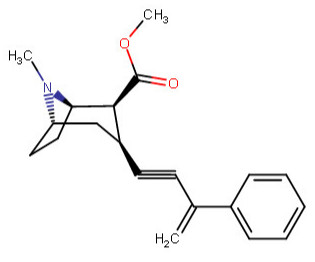
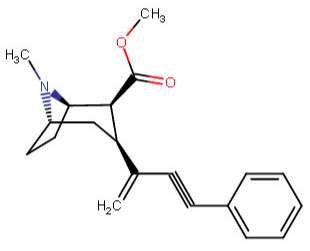
Anywho, at home I drew like over 60 permutations of the above, and I began to legitimately wonder which were wholly fiction on our environment here on Earth. I was going to make a chart with them all, and number them so someone could just call out the numbers of those which do not work in real world physics, like a strange game of cocaine analog bingo.
I may still do that if this doesn't get my question satisfactorily answered.
R1-3: any combination of H (not a het.at., but i.e. no carbon bond) a single methylene unit (planar, beta or alpha) a double bond, or a triple bond (where it doesn't make more than four by being adjacent to a double, of course)

There's no oxygens, but being pure carbon (besides the number of bonds) are there any rules I am breaking with certain combinations (rules like for instance possible with a heteroatom: 'keto–enol tautomerism') of the above?
I ask because of the drastic difference between 224a and 224d with binding; with the incredible selectivity of binding versus uptake of 224c, why weren't extra-carbon bonds tried along it in differing combinations?
I'll show some individual instances besides my R-substitution image above:



^^This one feeling like it is violating something, is it?^^


Anywho, at home I drew like over 60 permutations of the above, and I began to legitimately wonder which were wholly fiction on our environment here on Earth. I was going to make a chart with them all, and number them so someone could just call out the numbers of those which do not work in real world physics, like a strange game of cocaine analog bingo.
I may still do that if this doesn't get my question satisfactorily answered.