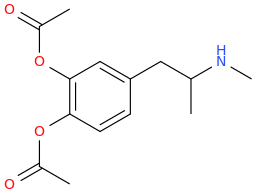
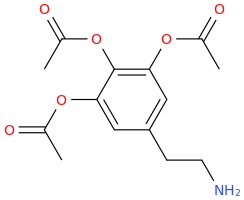
serotonin2A
Bluelighter
- Joined
- Sep 13, 2014
- Messages
- 1,354
The metabolite isn't intend to be excreted because it isn't a xenobiotic compound. That compound, dopamine, is endogenous and so there is a wholly different regulatory system for that and like neurotransmitters.
I don't think you can really distinguish the action of COMT on dopamine versus exogenous catecholamines. Xenobiotic compounds with catechol groups can also be metabolized by COMT.