LucidSDreamr
Bluelighter
- Joined
- May 23, 2013
- Messages
- 7,314
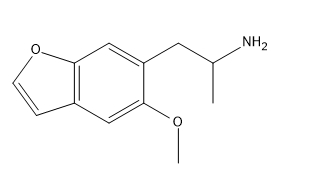
I have no interest in trying it as I can not do stimulants or psychs at all do to health reasons and I always hated stims anyways and psychs were more a curiosity in my youth but not exactly fun to me.
Something sparked my interest about it though.
Last edited: