Feretile
Bluelighter
- Joined
- Feb 2, 2022
- Messages
- 361
On page 242 of 'Opiates' by Lenz et al. is a QSAR of phenanthracine opioids. It suggests that the addition of an -OH at the 14 position and the replacement of the N-methyl with an N-2-arylethyl or (better) an N-2-(S)-hydroxy-2-phenylethyl moiety increases potency by a factor of 8 (2-phenylethyl) to a factor of 85 (2-(S)-hydroxy-2-(2-furyl)ethyl as compared to the parent compound (levorphanol - itself x10 morphine).
But there is a problem. The potency of compounds with both a 14-OH and an N-2-arylethyl is in doubt. Some papers suggest an increased potency when the 14-OH is present whereas other suggest a reduction in potency. I suggest that the reduced potency is because these examples possess some antagonist activity.
For those who do not know, the reason that certain N-substitutions possess antagonist activity is because of their conformation. In the 1960s a series of studies were carried out in which quaternary salts of morphine were produced. morphine allylbromide was the most telling. If you think about it, the N has 4 bonds and so their are 2 possible isomers. While these quaternary salts bear a formal charge and cannot pass the brain-blood barrier, in vitro studies using opiate receptors allowed the binding and activity to be studied. It turned out that one of the 2 isomers was an antagonist, the other an agonist.
What they concluded was than in minimum-energy conformation, N-allyl, N-methylcyclopropyl and to a lesser extent N-methylcyclobutyl and 2-methylpropenyl (as seen in pentazocine) would conform to the antagonist form.
Now, their is no suggestion that the studies into the levorphanol, 14-OH levorphanol & their N-2-arylethyl derivatives as well as hydromorphone, oxymorphone and their 2-arylethyl derivatives LOOKED for the conformation, but when potency would be expected to be significantly higher if the 14-OH was present, in all cases potency was the same or lower than the examples without the 14-OH.
I do realise that it's a minor point but it's a good example of the 'magic methyl' nature of medicinal chemistry.
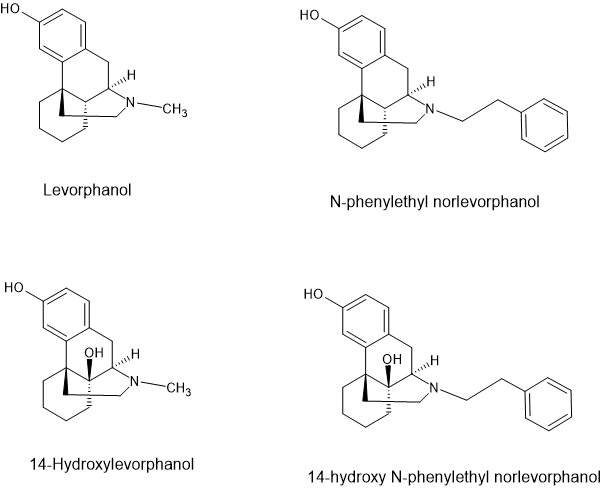
BTW I feel comfortable posting these because in spite of the fact that R. Grewe's original synthesis has been improved, levorphanol is still 11 or 12 steps from commercially available chemicals. As for 14-OH levorphanol, well the ONLY example in use is the N-methylcyclobutyl AKA butorphaol and in spite of Bristol Mayer spending a decade perfecting the synthesis, it's still 14 steps with a 4% overall yield. Safe to say, it's NEVER going to be made.
But there is a problem. The potency of compounds with both a 14-OH and an N-2-arylethyl is in doubt. Some papers suggest an increased potency when the 14-OH is present whereas other suggest a reduction in potency. I suggest that the reduced potency is because these examples possess some antagonist activity.
For those who do not know, the reason that certain N-substitutions possess antagonist activity is because of their conformation. In the 1960s a series of studies were carried out in which quaternary salts of morphine were produced. morphine allylbromide was the most telling. If you think about it, the N has 4 bonds and so their are 2 possible isomers. While these quaternary salts bear a formal charge and cannot pass the brain-blood barrier, in vitro studies using opiate receptors allowed the binding and activity to be studied. It turned out that one of the 2 isomers was an antagonist, the other an agonist.
What they concluded was than in minimum-energy conformation, N-allyl, N-methylcyclopropyl and to a lesser extent N-methylcyclobutyl and 2-methylpropenyl (as seen in pentazocine) would conform to the antagonist form.
Now, their is no suggestion that the studies into the levorphanol, 14-OH levorphanol & their N-2-arylethyl derivatives as well as hydromorphone, oxymorphone and their 2-arylethyl derivatives LOOKED for the conformation, but when potency would be expected to be significantly higher if the 14-OH was present, in all cases potency was the same or lower than the examples without the 14-OH.
I do realise that it's a minor point but it's a good example of the 'magic methyl' nature of medicinal chemistry.

BTW I feel comfortable posting these because in spite of the fact that R. Grewe's original synthesis has been improved, levorphanol is still 11 or 12 steps from commercially available chemicals. As for 14-OH levorphanol, well the ONLY example in use is the N-methylcyclobutyl AKA butorphaol and in spite of Bristol Mayer spending a decade perfecting the synthesis, it's still 14 steps with a 4% overall yield. Safe to say, it's NEVER going to be made.
