- Joined
- Aug 16, 2019
- Messages
- 4,688
N&PD Moderators: Skorpio | thegreenhand
Idk what SMILES meansI'm lazy, so send me the SMILES of it and I'll look it up for you.
You mean the numbers for example like c4h11 type numbers? If so I couldn't find them.Idk what SMILES means
Text notation of the molecule.Idk what SMILES means
It won't let me on my phone, but I posted a pic of it and another one.Text notation of the molecule.
Just go redraw it here: http://swisstargetprediction.ch/ and send us the link for what you get.
Text notation of the molecule.
Just go redraw it here: http://swisstargetprediction.ch/ and send us the link for what you get.
Ok I got it to work, I made a new one though CCC(CCC(C)=O)(C1=CC=[Cl]C=C1)C1=CC=[Br]C=C1Text notation of the molecule.
Just go redraw it here: http://swisstargetprediction.ch/ and send us the link for what you get.
I made a new oneI'm lazy, so send me the SMILES of it and I'll look it up for you.
Strong likelihood it doesn't do much, but here you go:I found out how to do it, but here is prodopalol.
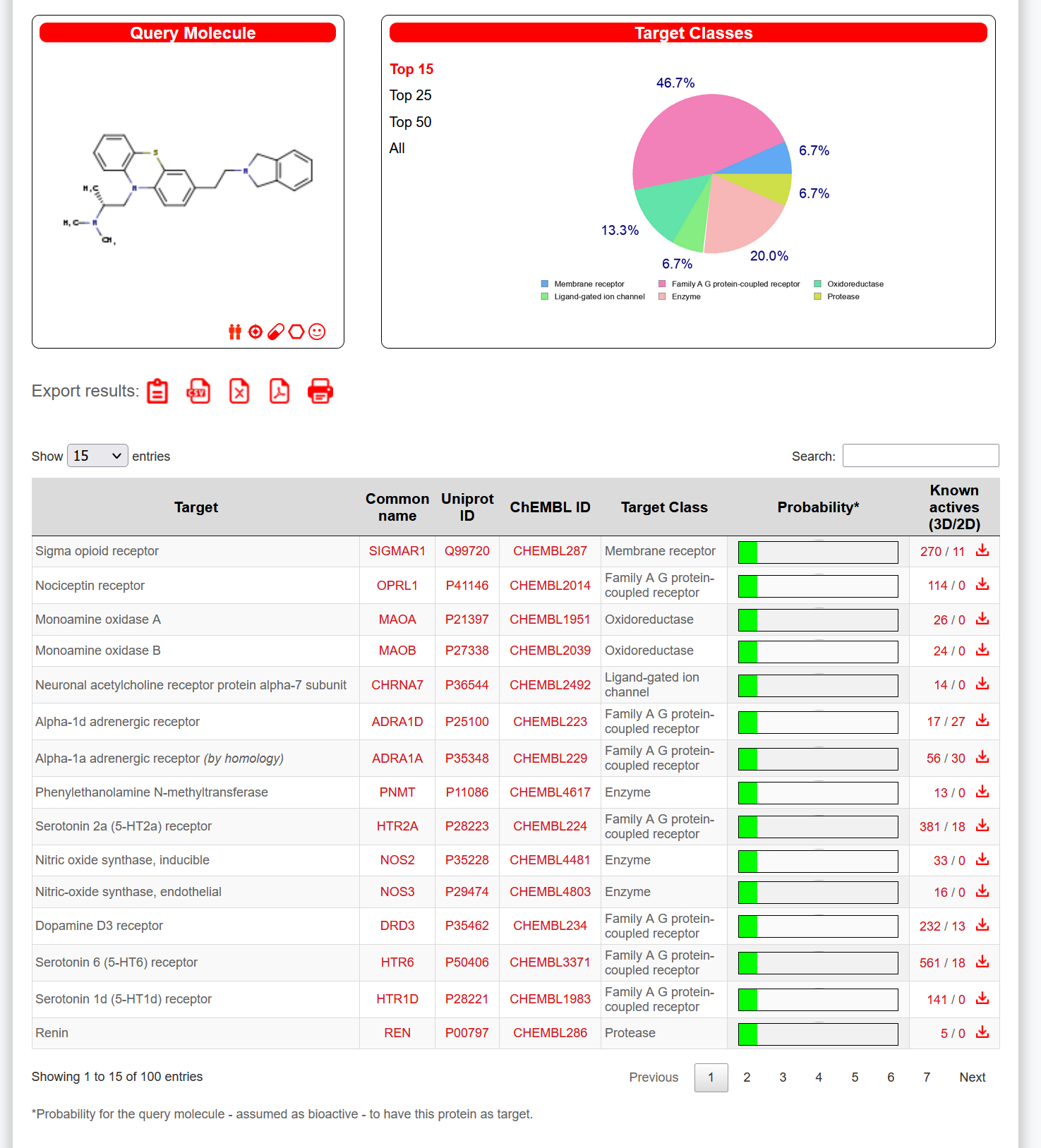
That one does absolutely nothing, are you just making random molecules here?I made a new one
CCC(CCC(C)=O)(C1=CC=[Cl]C=C1)C1=CC=[Br]C=C1
It needs to be SMILES, not 10th grade Chemistry notation where you have no clue as to what the arrangement of the molecule is.C10H13BrClN
Oh I tried this part of the website with other molecules I made, but didn't come up with as many dopaminergic type receptors, or receptors that would cause any recreational effects (sorry I'm literally a highschool drop out with 0 knowledge on chemistry at all besides things I've read on the Internet, which I feel like I know quite a bit for not being taught at all whatsoever). Well some of them did, but it was like one serotonin receptor, Norepinephrine, and the rest were random receptors I've never heard of. So I was using the website right and that some of them just bind to other random receptors I know nothing about, and didn't even know existed?Strong likelihood it doesn't do much, but here you go:

Do you know what receptors it binds to?Wee! Great!
So now the random molecule wont spread around all over the topics!
Let me start this (supposedly fun?) topic by this one:
(Please vote a name for it!)

I think benzos need some saturation in the diazepine ring to have the right shape to fit into the binding pocket (also another nitrogen for h bonding). The molecule above looks pretty floppy. I'd also expect that position of the methoxy to reduce affinities broadly, never seen a lot of bulk there.I haven't named this one yet, or got the chance to check out the receptors it would or could bind to. If anyone can throw in that information that would be cool.
So you think it looks like a benzodiazepine? I wonder what I should call it?I think benzos need some saturation in the diazepine ring to have the right shape to fit into the binding pocket (also another nitrogen for h bonding). The molecule above looks pretty floppy. I'd also expect that position of the methoxy to reduce affinities broadly, never seen a lot of bulk there.
Run it through Swiss though and see what it says.
Honestly you can pick up a lot about bzd sar just looking through different Wikipedia articles, seeing what substituants give potency or selectivity.
Likely there is published work that compiles a lot of qsar data, you are on your own for finding that for now- I'm pretty slammed today for time.
