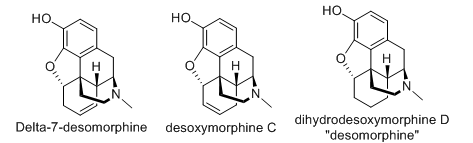
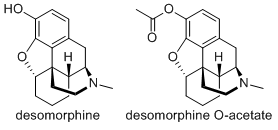
Yes indeed, I don't see why not. If you are familiar with the line drawings used to denote chemical structures, presence of "OH" or "HO" groups are sometimes indicative of an alcohol group. (as long as some exceptions are excluded, like carboxylic acids or peroxy compounds) Those are the locations that acetate groups are attached onto, increasing fat solubility & therefore potency). Desomorphine has one of these, at the 3-position, attached to the topmost phenyl ring.
(4R,4aR,7aS,12bS)-3-methyl-2,3,4,4a,5,6,7,7a-octahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-9-yl acetate would be the IUPAC name. Just rolls right off the tongue,
I do not know whether this compound would pose any benefits over "normal" desomorphine. Heroin is useful because morphine is a bit of a poorly behaved drug, by switching to the ester you produce a more potent compound that can be given orally in smaller doses than morphine would need.
Usually, the phenolic 3-position hydroxy is required to be present and capable of hydrogen bonding to produce agonist activity at opioid receptors. This is why codiene and ethylmorphine (3-O-methoxy / 3-O-ethoxy morphine) is much less effective than morphine, yet heterocodeine (6-O-methoxymorphine) that has the methyl group moved to the other alcohol is
more potent than morphine!
Also, even though it is known that heroin produces strong narcotic effects, it is known that purified heroin is actually not very good at binding to the opioid receptors in the absence of esterase enzymes which would cleave the acetate esters. (nor is 3-O-acetylmorphine) It just so happens that your body will remove tbe 3-O-acetate basically instantly, so the effects of heroin are actually more correctly said to be from 6-monoacetylmorphine and morphine.