Fertile
Bluelighter
- Joined
- Mar 31, 2022
- Messages
- 1,627
Buccinazine has been around since the 1960s and is supposed to be about ⅓ the potency of morphine.
A quick search of the patents shows that the team behind buccinazine went on to develop azaprocin (x30 the potency of buccinazine). But they also discovered that a simple substitution increased the potency by a factor of 2.5 i.e. addition of a p-NO2 i.e.
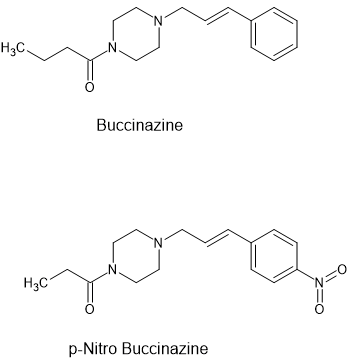
It seems an obvious development. But if you seek a novel scaffold:
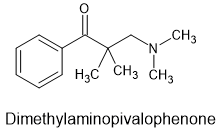
Now I have found the above as a fragment of more potent opioids but unlike the phenylpiperidines, phenylbenzamides or others - their is no obvious way to increase potency and/or duration.
A quick search of the patents shows that the team behind buccinazine went on to develop azaprocin (x30 the potency of buccinazine). But they also discovered that a simple substitution increased the potency by a factor of 2.5 i.e. addition of a p-NO2 i.e.

It seems an obvious development. But if you seek a novel scaffold:

Now I have found the above as a fragment of more potent opioids but unlike the phenylpiperidines, phenylbenzamides or others - their is no obvious way to increase potency and/or duration.
