Institute of Clinical Medicine, University of Oslo
Psychedelics as a novel approach to treating autoimmune conditions
Caitlin Thompson, Attila Szabo | December 2020
With a rise in the incidence of autoimmune diseases (AiD), health care providers continue to seek out more efficacious treatment approaches for the AiD patient population. Classic serotonergic psychedelics have recently been gaining public and professional interest as novel interventions to a number of mental health afflictions. Psychedelics have also been shown to be able to modulate immune functions, however, while there has been great interest to researching into their psychotherapeutic applications, there has so far been very little exploration into the potential to treat inflammatory and immune-related diseases with these compounds. A handful of studies from a variety of fields suggest that psychedelics do indeed have effects in the body that may attenuate the outcome of AiD. This literature review explores existing evidence that psychedelic compounds may offer a potential novel application in the treatment of pathologies related to autoimmunity. We propose that psychedelics hold the potential to attenuate or even resolve autoimmunity by targeting psychosomatic origins, maladaptive chronic stress responses, inflammatory pathways, immune modulation and enteric microbiome populations.
1. Introduction
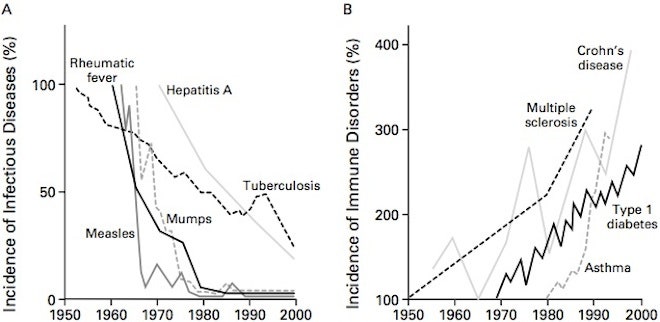
The incidence of autoimmune diseases (AiD) has been steadily increasing in the last decades, as reported by The National Institute of Health (NIH) and other sources. There are currently over 100 defined autoimmune-related diseases affecting roughly 50 million Americans, or 20 % of the population according to the American Autoimmune Related Diseases Association (AARDA). The NIH also calculates that over $100 billion in health care costs is spent annually in the United States on treating those with autoimmune diseases, compared to cancer which has an estimated cost of $50 billion annually. It is conservatively estimated that 80 % of those affected by AiD are women. There are several theories for why women are more susceptible to AiDs, but known reasons remain unclear. Different theories for the increased prevalence of AiDs include overuse of antibiotics, increase in environmental toxin exposure, increased caesarian births, reduced breast feeding, improvement of diagnostic tools, increased awareness of AiDs and increased societal stressors.
Autoimmunity is defined as the loss of tolerance to self-antigens in which the immune system attacks healthy tissues in an individual. While there are different characteristics of expression and tissues affected amongst the autoimmune disorder types, there are many shared mechanisms of action and potential origins. Many diseases that were initially considered to be unrelated to autoimmunity are now being reexplored as autoimmune-related, especially in the field of psychiatry. This includes major depressive disorder (MDD), schizophrenia, Parkinson’s disease, Alzheimer’s disease and Amyotrophic Lateral Sclerosis.
While the causes of AiDs are controversial, they appear to be multifactorial with many facets that contribute to their development such as genetic predisposition, environmental triggers, and psychosocial stress. There is strong evidence that traumatic experiences such as Adverse Childhood Events (ACEs) and prolonged stress may contribute to the development of an AiD. AiDs often encompass a variety of symptoms and features. Common characteristics observed among the various types of AiD include increased vascular and epithelial (
e.g. intestinal) permeability, chronic inflammation, the presence of chronic infections, dysregulated Hypothalamic-pituitary-adrenal axis (HPA axis), mitochondrial dysfunction, and microbiome dysbiosis. Symptoms can manifest as chronic fatigue, allergies, psychiatric and mood disorders, pain, rashes, GI distress, poor cognition and more.
Currently, biologics and immunosuppressive drugs are the primary treatment tools for autoimmune disorders along with non-steroidal anti-inflammatory drugs, steroids and antidepressants. However, immunosuppressant and biologic medications designed to resemble antibodies, receptors and other immunological factors can result in an immune compromised state, putting the affected population at risk for serious infections or immune-related illnesses. Although existing biological drug approaches show evidence for producing positive treatment outcomes in some individuals, the increased risks for infection, malignancy and cardiovascular conditions, as well as contradicting results in efficacy studies have prompted interest in exploring other approaches to treating AiDs.
2. The immunopharmacology of psychedelics
2.1. The link between autoimmunity and mental disorders
The comorbidity between autoimmune conditions and mental and mood disorders, such as MDD, anxiety, schizophrenia, and bipolar disorder has become apparent in the last two decades. There is a higher risk of developing clinical depression or mood disorders if one has been diagnosed with an autoimmune condition. While there is certainly argument that the burden of having an autoimmune condition could contribute to MDD, researchers suggest that depression and anxiety symptoms could perhaps be a result of autoimmune mechanisms and resulting inflammation occurring in the nervous system, or
via dysregulated inflammatory cytokine loops between peripheral and brain-resident immune cells. Potentially by their immunomodulatory activity and in part through the mobilization of cell-intrinsic neuroprotective mechanisms, psychedelics may represent a promising intervention for autoimmune-related depression and other mental illness.
2.2. Pharmacology of psychedelics substances
Psychedelic compounds have recently made their way back into mainstream medical research. Psychedelic compounds such as LSD, psilocybin, DMT, ibogaine and mescaline have been of great interest based on their apparent high efficacy for treating mental afflictions and their impressive safety profile. Psychedelic drugs were popularized in the 1960s by researchers such as Timothy Leary, Ram Dass, Terrence McKenna and other enthusiasts. Between the 1960’s and the 1980’s a number of scientific studies were being conducted on psychedelic substances including biochemical, pharmacological and psychological studies. Therapists were experimenting with using psychedelics as adjuncts to various psychotherapy methods. Due to radical activism and drug propaganda, psychedelics were scheduled as Schedule I substances by the Controlled Substances Act in 1970 and were made illegal to consume, possess or distribute. Contradictory to this scheduling decision, research currently suggests that class psychedelic compounds do not show evidence of addiction potential and exhibit anti-addiction properties while also appearing to be generally physiologically safe, non-toxic and exhibiting minimal individual and societal harms. This prohibition resulted in an abrupt halt in research on psychedelics. In the early 90’s there was the start of a new renaissance in psychedelic research with the work of Risk Strassman on intravenous DMT administration in humans. Psychedelic research currently is a rapidly accelerating field in science, psychology and medicine.
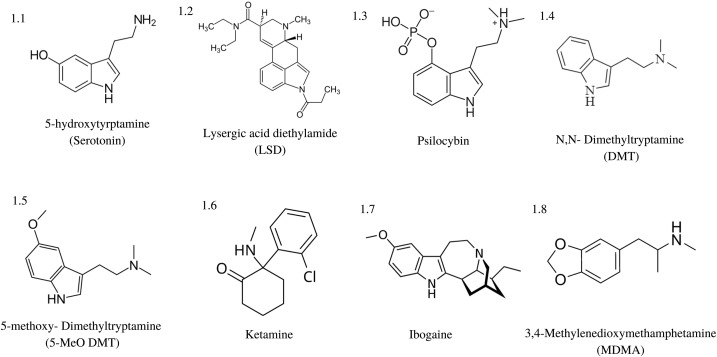
“Classic” psychedelics generally refer to LSD, psilocybin, mescaline, ibogaine, DMT, and 5-MeO-DMT, and are typically defined by their indole-containing molecular structure as illustrated in
Fig. 1. Non-classic psychedelics such as ketamine and MDMA may have some similar receptor activity and hallucinogenic properties, but lack this fundamental indole structure. Classic psychedelics typically and predominately exhibit agonistic activity at various serotonin (5-hydroxytryptamine, 5-HT) receptors, with the most commonly shared feature being their 5-HT2A receptor agonist binding. The classic psychedelics vary in their receptor binding properties but overall show binding affinity for a variety of receptors including 5-HTR subtypes. LSD [Fig. 1.2] is known to bind to 5-HT1A, 1B, 1D, 1E, 2A, 2B, 2C, 5A, 6, and 7 as well as dopaminergic receptors subtypes D1 and D2. Psilocin, the active metabolite of psilocybin [Fig. 1.3], binds to 5-HT1A, 1D, 2A, 2C but does not bind to dopaminergic receptors. Mescaline exhibits the highest binding affinity to the 5-HT2A and 2C receptors.
Fig. 1. Structure of classic psychedelic compounds. Many classic psychedelics share an indole structure
within their molecular makeup, often characterizing them as serotonin analogues and agonists.
DMT [Fig. 1.4] and its psychopharmacologically more potent analogue 5-MeO-DMT [Fig. 1.5] bind to the 5-HT1A, 1B, 1D, 2A, 2B, 2C, 5A, 6 and 7 receptors, as well a number of iontropic and metabotropic glutamate receptors, dopamine, acetylcholine, Trace-Amine-Associated Receptors (TAAR), and the opioid-like receptor sigma-1 (Sig1R). DMT is found in trace amounts in most plant, animal (including humans) and fungal organisms with some plants, such as the
Acacia and
Mimosa genera, containing more substantial amounts. It is one of the components in the traditional Amazonian brew ayahuasca, which combines DMT-containing plants, such as
Psychotria viridis with the Monoamine Oxidase Inhibitor (MAOI)-containing
Banisteriopsis caapi (ayahuasca) vine to potentiate a psychoactive effect from the oral administration of DMT
Fig. 2. The ayahuasca vine contains harmala alkaloids, such as harmine, harmaline and tetra-harmaline that act as MAOIs in the ayahuasca brew. Typically, the DMT contained in the ayahuasca brew is considered the psychoactive constituent, however a ritual called
Natemamu does exist in the Shuar culture in which only the
B. caapi vine is used, suggesting that at certain doses, the MAOIs from the ayahuasca vine on its own can create a hallucinatory experience.
Fig. 2. Naturally occuring psychedelics. (2.1) Banisteriopsis caapi, also known as the ayahuasca vine, contains monoamine-oxidase inhibitors which, when combined with a DMT-containing plant, such as
Psychotria viridis, make up the psychoactive brew known as ayahuasca.
(2.2) Incilius alvarius, commonly known as the Colorado River Toad, excretes 5-MeO-DMT in its defensive secretions.
(2.3) Lophophora williamsii, or Peyote, has been used as a sacrament in traditional Native American ceremonies for thousands of years.
(2.4) Psilocybe cubensis is a common species of psilocybin mushrooms. Images sourced from
Wikipedia.
5-MeO-DMT is also found in many plants and animals including in the Amazonian shamanic snuff called “yopo” (
Anadenanthera peregrinaa), mammalian tissues and within the defensive secretion of the Colorado River Toad (
Incilius alvarius)
Fig. 2. Because of the degradation of the DMT and 5-MeO-DMT molecules by MAO in the digestive tract, the most common way of ingesting these substances is through inhalation
via vaporization. There is a long history of mixing DMT with MAOIs in various South American traditions, however, there does not appear to be any traditional practices mixing 5-MeO-DMT with MAOIs as it appears to be potentially dangerous due to higher 5-HT binding affinity and disparate metabolic degradation pathways.
Some “not-so-classic” psychedelic compounds, such as ketamine and ibogaine have complex pharmacology that is not 5-HT dominant. Ketamine [Fig. 1.6] exhibits non-competitive NMDA receptor antagonism, while displaying monoaminergic release as well as cholinergic, adrenergic, μ- and δ-opioid receptor binding. Ibogaine [Fig. 1.7], an alkaloid from the
Tabernanthe iboga shrub, has a highly promiscuous binding affinity for the 5-HT and dopamine (DA) transporters, non-competitive antagonism for several types of nicotinic AChR, NMDA Glu, μ-, δ- and к- opioid, Sig1R and Sig2 receptors. MDMA [Fig. 1.8], while generally referred to as an empathogen rather than a true psychedelic, releases presynaptic vesicular monoamines 5-HT, DA and norepinephrine (NE) while inhibiting their reuptake into the presynaptic cell, flooding the synapse. It does however have some agonistic binding affinity to 5-HT2A receptors like classic psychedelics, as well as muscarinic receptor 1, and the H1 histamine receptor.
Although at this point there has been increased exploration into the use of psychedelics as tools for the treatment of MDD, post-traumatic-stress-disorder (PTSD), addiction, eating disorders, anxiety and other mental disorders, there has been very little investigation into the immune modulating and anti-inflammatory effects of psychedelics. Biochemical and anecdotal evidence suggests that psychedelics could provide a novel treatment approach to AiDs and other immune and inflammatory-related diseases. It is likely that psychedelics can attenuate autoimmunity
via a number of different mechanisms. Psychedelics may directly and indirectly target a number of physiological factors and resulting dysfunctions in AiDs. This review covers the current evidence that psychedelics may offer therapeutic solutions and potential modalities for the attenuation of AiDs.
2.3. Inflammation and immune modulation
In AiD, rampant chronic inflammation is a keystone feature. Elevated levels of cytokines and their dysfunctional regulation involving interleukin(IL)-6, IL-1β, IL-17, tumor necrosis factor-α (TNF)-α, IL-12, interferon(IFN)-γ and others are a shared feature observed in many AiDs including Rheumatoid Arthritis (RA), Systemic Lupus Erythematosus (SLE), systemic sclerosis, and Sjögren's syndrome. Lipopolysaccharide (LPS) is an inflammatory component of the cell wall of Gram-negative bacteria that can induce inflammation and excessive oxidative stress that may play a role in elevated inflammatory biomarkers seen in those with AiD diseases. Those with AiD display auto-antibodies and yet also display immunodeficiency. Immune modulation appears to be a feasible target in approaching the treatment of AiD.
While the area of research is quite underdeveloped, there is some evidence that psychedelic compounds have anti-inflammatory and immune-modulating effects
Table 1. For instance, LSD displays the ability to suppress the proliferation of B-lymphocytes, as well as the production of the pro-inflammatory cytokines IL-2, IL-4, and IL-6 in
in vitro splenic lymphocytes derived from 6−8 year old female B6C3F1 rats at concentrations of LSD between 1–100 μM. The same study observed that administered doses of LSD between 0.001−0.1 μM in rat lymphocytes increased the number of Natural Killer (NK) cells, while concentrations of 100 μM suppressed NK cell production. In another study, the substituted amphetamine DOI, was shown to reduce TNF-α levels in 10 week old adult male C57BL/6 J mice
via agonism of the 5-HT2A receptor in doses ranging from 0.01 μM/kg to 10μM/kg. Furthermore, in a recent study the same group expanded the previously observed anti-inflammatory effects of 5-HT2A agonism to additional psychedelics in a rat model of lung inflammation.
Table 1. Receptor modulatory and physiological effects of psychedelics.
�
One human study where ayahuasca was given to healthy volunteers recorded blood levels of lymphocytes and found a decrease in CD4 and CD3 cells, and an increase in NK cells. This was compared to controls and subjects treated with D-amphetamine. Another, more recent human study reported significant decrease in C-reactive protein levels in the blood in both healthy and depressed volunteers, but no significant reduction in IL-6 blood levels. In addition, in a human intramuscular ketamine study, levels of proinflammatory markers, including C-reactive protein (CRP), IL-6, and TNF-α, were examined prior to initiation of test infusions, 40 min post-infusion, 240 min post-infusion, and on day 3 and day 7 post-fusion in patients who received 0.5 mg/kg or 0.2 mg/ kg of R/S-ketamine hydrochloride. Acute reductions of IL-6 and TNF-α were found in the 0.5 mg/kg group between 40 min. and 240 min post-infusion, but not in day 3 or day 7. Reductions in pro-inflammatory cytokine levels were directly correlated with depression scores, except on days 3 and 7 when depression scores improved despite cytokine levels returning to pre-infusion baselines. Interestingly, a different study obtained contradicting results showing an increase in IL-6 post-infusion and no correlation of cytokine levels with anti-depression outcome, suggesting that more studies need to be conducted to understand the complex systemic immune effects of ketamine and its metabolites.
There is substantial literature on the 5-HT system and its complex inflammation and immune-regulating abilities in tissue specific manners. Functional studies showed that 5-HT modulates the release of IL-1beta, IL-6, IL-8/CXCL8, IL-12p40 and TNF-α, while it has no effect on the production of IL-18 and IFN-gamma in LPS-stimulated human blood monocytes. 5-HT can also modulate human macrophage polarization and dendritic cell functions, and can contribute to the maintenance of an anti-inflammatory state
via 5-HT2B and 5-HT7 receptor binding. Given the role of the 5-HT system in immune-modulation and inflammatory properties, it is highly likely that there are undiscovered immune and inflammatory effects from exposure to psychedelic compounds due to their serotonergic activity. In the previously mentioned study where DOI was observed to reduce TNF-α in mice, in groups where the 5-HT2A antagonist drug M100907 was administered, the reduction in TNF-α was not observed supporting the theory that the 5-HT2A receptor and the 5-HT system as a whole may be fundamental to creating downstream immunological and inflammatory-regulating effects.
In addition to the 5-HT receptors, DMT and 5-MeO-DMT also have high binding affinity for the Sig1R. The Sig1R plays a fundamental role in the regulation of different cellular mechanisms such as mitochondrial function, apoptosis, proliferation, and neuroprotection. Sig1R also modulates inflammatory and immune responses by regulating the activation of the transcription factors nuclear factor kappa B (NF-кB) and several mitogen-activated protein kinases (MAPKs). Both NF-кB and MAPKs are important regulators of gene transcription involving immune responses and the production of inflammatory cytokines.
Abnormal Sig1R functions have been implicated in a number of psychiatric and inflammatory-related conditions such as MDD, Alzheimer’s disease, Parkinson’s disease, cardiovascular disease, immune reactions, and proliferation of cancer cells. Activation of the Sig1R is also pivotal in facilitating stress responses. Dysregulated NF-кB and MAPK activity patterns have been implicated in a number of AiD pathologies. One study showed evidence of suppression of pro-inflammatory NF-кB cytokine production by administration of harmine, a component of the ayahuasca vine,
via intraperitoneal injection at doses of 25 and 50 mg/kg, in response to treatment with LPS in Male Kunming mice to induce acute kidney injury. Another study found that harmine inhibited tumor necrosis factor-α (TNF-α)- and LPS-induced NF-κB transactivity and nuclear translocation in mouse macrophage RAW 264.7 cells when treated with concentration between 2.5–25 μM. 5-MeO-DMT was also found to inhibit the NF-κB signaling pathway in human cerebral organoids exposed to concentrations between 23 nM to 7.11 μM, and examined for proteomic analysis
via mass spectrometry. In other
in vitro and animal
in vivo studies, DMT and 5-MeO-DMT have been shown to increase anti-inflammatory IL-10 while decreasing the levels of the pro-inflammatory IL-1β, IL-6, TNF-α, and the chemokine CXCL8/IL-8 in human monocyte-derived dendritic cells at concentrations of 100 μM. DMT also exhibited considerable neuroprotective and anti-neuroinflammatory effects in a rat model of stroke where male Wistar rats received an intra-peritoneal (IP) bolus of 1 mg/kg-body weight (bw) DMT followed by a maintenance dose of 2 mg/Kg-bw/h delivered over 24 h after induced transient middle cerebral occlusion.
Furthermore, inhaled 5-MeO-DMT has recently been reported to decrease salivary IL-6 levels in a study involving a small group of human subjects. There results suggest that serotonergic psychedelics may emerge as potential candidates in the treatment of autoinflammatory and autoimmune conditions
Table 1. Since pharmaceutical grade DMT and other tryptamine analogs designed for human clinical trials are already available or will soon enter the market, testing the
in vivo physiological effects of serotonergic psychedelics in humans is now closer than ever before.
2.4. Trauma and emotional effects
A number of studies have suggested that significant or prolonged stress, especially in early childhood, contributes to the development of a number of diseases including AiD. The severity of childhood trauma can be clinically assessed by scoring ACEs (Adverse Childhood Experiences). Many studies have found strong correlations between ACEs and the statistical risk of developing an AiD including studies looking at associations between childhood stress and RA, SLE, and fibromyalgia.
The downstream physiological detriment due to stress and vagal nerve disruption is well documented in the literature and appears to play a significant role in the development of an AiD. Psychological stress and psychoemotional trauma can severely compromise the immune system leading to increased risk of chronic infections and gut dysbiosis. Psychosocial stress increases inflammatory cytokines, oxidative stress, glutamate excitotoxicity, inhibits effective digestion and nutrient absorption and contributes to HPA axis dysfunction. Stress has been found to increase intestinal permeability which can lead to food sensitivities and LPS-induced inflammation due to gut-blood transepithelial bacterial translocation (“Leaky Gut syndrome”). All of these inflictions are hallmark characteristics of patients with AiD, further supporting the hypothesis that stress may play a significant role in AiDs.
The psychedelic compounds psilocybin, LSD, MDMA, and ayahuasca are currently being explored for their promising potential to assist in treating trauma-derived illnesses such as PTSD, depression, and addiction. The research with MDMA assisted psychotherapy for PTSD is currently the furthest along in its drug development and approval process by the FDA than any other psychedelic-related compound with psilocybin, cannabis and ayahuasca following closely behind. MDMA assisted psychotherapy has received breakthrough status by the FDA, accelerating its entry into mainstream medical practice. The MDMA therapy for PTSD protocol developed by MAPS, consisting of two MDMA-assisted therapy sessions with the patient receiving 125 mg of MDMA orally in conjunction with non-directive psychotherapy, along with preparatory and post non-MDMA therapy sessions, has so far exceeded all existing treatments for PTSD in its symptom remission statistics. Although the classic psychedelics have not been clinically established as strongly for PTSD as MDMA has, there is evidence that they also hold potential for stress and trauma-oriented applications. There are several human and non-human primate studies that suggest that the DMT-containing ayahuasca brew has modulatory effects on salivary cortisol response and plasma cortisol in both healthy normals and in individuals with treatment-resistant MDD. Thus, potential neuroendocrine-immune modulation is one of the recently proposed mechanism for the rapid anti-depressant effects of ayahuasca in those with MDD.
Given that trauma and chronic sympathetic activation are prevalent among those with AiD, the therapeutic outcome of psychedelic-assisted psychotherapy could resolve or improve stressful psychological states that cause or contribute to physiological outcomes seen in the majority of AiD patients.
2.5. Glutamate excitotoxicity
Those with autoimmune conditions, particularly those affecting the nervous system, are more likely to have increased glutamate (Glu) binding sensitivity and post-synaptic glutamate levels which can result in cytoxicity to the nerve cell. The term glutamate excitotoxicity is used to describe the cytotoxic effects of excessive and uncontrolled release and binding of glutamate to post-synaptic neurons. Hypersensitization of Glu receptors due to cytokine-mediated transcriptional changes that affect depolarization gradients potentially play a role in this glutamate-induced cytotoxic phenomenon. Excitotoxicity appears to be implicated in a number of brain related diseases, including multiple sclerosis, depression, addiction and neurodegenerative diseases. During glutamate-excitotoxicity, excessive stimulation of the Glu receptors resulting in action potentials to be propagated through the neuron in an uncontrolled manner. This in turn activates the influx of Ca2+ ions into the cytoplasm where they may induce mitochondrial respiration and the release of reactive oxygen species (ROS). Under normal circumstances, the generated ROS are cleared from the cell and transmembrane ion gradients are restored quickly after glutamate transmission. However, in activation-induced excitotoxic cascades, the permeability transition pore (PTP) is opened and mitochondrial dysfunction occurs due to increased oxidative stress, abnormal ATP synthase activity and inhibited mitochondrial respiration. Chronic excitation of the Glu receptors can therefore contribute to oxidative stress, which may result in damage to neural structures or apoptotic cell death. Excitotoxicity from excessive Glu receptor binding may result in changes in cortical, amygdala, and hippocampal density leading to atrophy and shrinkage of brain regions.
While not a classic psychedelic compound, the dissociative anesthetic drug ketamine displays an ability to protect against glutamate-mediated excitotoxicity by non-competitively blocking the NMDA glutamate receptor. It is unclear if inhibition of the NMDA Glu receptor is the fundamental mechanism for the neuroprotective properties or if this, in turn, redirects glutamate to bind to other Glu receptors such as AMPA, Kainate and metabotropic Glu receptors. It appears that blocking the NMDA GluR, while flooding AMPA and metabotropic Glu receptors, paradoxically enhances Glu transmission in the prefrontal cortex in a way that is synaptogenic and potentially restorative for appropriate Glu receptor sensitivity. This could potentially be one mechanism for how ketamine delivers such rapid anti-depressant effects. In theory, blocking or correcting an excitotoxic effect from glutamate may alleviate the oxidative stress and tissue damage to cells by reducing the ROS released
via action potentials.
Sig1R agonists such as dimemorfan, and dipentylammonium have shown neuroprotective mediation of glutamate excitotoxicity. Furthermore, DMT has been shown to possess potent neuroprotective effects
via the Sig1R in both human
in vitro and animal
in vivo studies. It may be possible that the psychedelic Sig1R agonists DMT and 5-MeO-DMT are also capable of mediating glutamate levels and receptor activity
via indirect transcriptional effects on cytokine production. Harmine, a MAOI contained in the ayahuasca vine has been found to increase Excitatory Amino Acid Transporter 2 (EAAT2) glutamate pump expression in the central nervous system, therefore raising the possibility of reducing glutamate toxicity
in vivo.
5-HT receptor activation also has modulatory effects on downstream Glu release and binding activity, in particular through activity at the 5-HT1A, 5-HT1B, 5-HT2, 5-HT3 and 5-HT6 receptors in various brain regions. Downstream Glu effects from 5-HT activity of psychedelic drugs appears to play a role in the neurogenic properties of psychedelic compounds. It is possible that classic psychedelics that act on the 5-HT system may have indirect mitigation of pathological glutamate transmission and receptor sensitivity.
2.6. BDNF and neuroplasticity
The classic psychedelics psilocybin, DMT, 5-MeO-DMT, LSD, the psychedelic ritual brew ayahuasca, as well as the non-classic psychedelics ketamine have shown potential in interacting with neurogenic pathways, such as Tropomyosin receptor kinase B (Trk-B) and the mammalian target of rapamycin (mTOR), in an equivalent manner to the neurotrophin protein brain-derived neurotrophic factor (BDNF), and in influencing neuroplasticity in
in vitro and
in vivo models of
Drosophila melanogaster, rats and humans
Table 1. There are conflicting results from studies measuring BDNF gene expression and protein levels in serum with some studies showing evidence of enhanced BDNF gene expression as a result of exposure to psychedelics (LSD) in
in vivo rat models, while others show no increase in gene expression but increase in BDNF protein levels
in vitro and
in vivo (serum) in rats. In studies, where the Trk-B selective antagonist ANA-12 was administered, the synaptogenic and neurogenic properties of psychedelics and BDNF are completely blocked. Similarly, treating rat cortical neurons with the drug rapamycin to inhibit mTOR also blocked the psychedelic-induced neuritogenesis suggesting that BDNF and psychedelics possess a shared mechanism for promoting neuritogenesis through an mTOR-related process of protein synthesis in synaptogenesis. It appears that this pathway is directly related to 5-HT2A receptor agonist activity of classic psychedelics, given that when ketanserin, a 5-HT2A antagonist, is administered the neuroplastic effects of DMT, LSD, and DOI are abrogated. Studies on ketamine’s effect on BDNF levels show increase of BDNF protein levels in the hippocampus but not an increase of BDNF mRNA. The increased BDNF levels were not sustained past 24 h after treatment, implying that BDNF interaction at TrK-B receptor sites prompts intracellular signaling that is responsible for the synaptic plasticity and anti-depressant effects.
One study that explored the neurogenic properties of indole-containing psychedelic compounds and ketamine in
Drosophila and rat
in vitro cortical neuron cell cultures used concentrations of each substance at 10 nM as the upper limit of each substance, with the exception of DMT, where 90 nM was used to simulate levels naturally occurring in rat brains. In
in vivo rat models using Sprague-Dawley rats 10 mg/kg and 1 mg/kg doses of DMT were administered
via injection and each dosage category produced similar responses in increasing density of dendritic spines. The results demonstrated that psychedelics can influence neuronal structure in vertebrates (Sprague-Dawley rats) and invertebrates (
Drosophila) in both
in vitro and
in vivo models suggesting an evolutionarily conserved mechanism. In a similar manner, in human studies a single-dose of ayahuasca increased BDNF serum levels in both depressed and control subjects. Human brain imaging studies have also shown grey matter density changes as thinning in the posterior cingulate cortex (PCC) and thickening in the anterior cingulate cortex (ACC) in long-term ayahuasca users suggesting modulated neural plasticity and structural changes as a result of regular ayahuasca consumption.
BDNF is fundamental for facilitating the repair, maintenance and survival of neurons. Through its Trk-B-dependent signaling, BDNF prompts the growth of new synaptic spines, dendrites and new whole neurons. BDNF signaling is fundamental in dictating brain tissue density and the formation and conservation of neural networks. Low expression of BDNF mRNA in brain, and protein levels in serum and plasma in humans have been linked to MDD, anxiety, schizophrenia, and neurodegenerative diseases. Given the prevalence of MDD and glutamate excitotoxicity in patients with autoimmune diseases, it is possible that the BDNF-upregulating qualities of psychedelics could attenuate the inflammatory and cytotoxic pro-oxidative effects of glutamate excitotoxity in autoimmune disease, resulting in increased survivability of neurons that may be susceptible to ROS damage.
2.7. Microbial aspects of the physiological effects of psychedelics
Chronic infections with bacteria, fungi and viruses are common in those with AiDs. Studies suggest that infection by the Epstein-Barr virus and other herpes family viruses are prevalent in those with AiD. There is also evidence that
Borrelia burgdorferi, the bacteria that causes Lyme disease, is also a common infectious agent in AiDs. Many patients with AiD display evidence of increased gut permeability, small intestinal bacterial overgrowth, and other signs of gut dysbiosis, which can be classified as chronic low-grade infections.
Constituents in psychedelic plants such as the ayahuasca vine (
Banisteriopsis caapi) and Peyote cactus (
Lophophora williamsii) display specific antimicrobial effects. Harmine and harmaline are beta-carboline alkaloids with MAOI properties in the
B. caapi vine. Studies have demonstrated
invitro anti-viral activity against the Herpes Virus Simplex 2 (HSV2) virus, a common infection in those with AiD, in HeLa, Vero, and Hec-1-A cells. The same alkaloids also appear to have antifungal properties which could perhaps be advantageous in an individual with a filamentous fungi overgrowth. Peyote extract has also shown broad antibacterial activity
invitro, in particular against 18 different penicillin-resistant strains of
Staphylococcus aureus. There may be mild antibiotic effects of some psychedelic plants which could potentially yield notable results in managing chronic or acute infections in AiD patients.
While there is evidence to suggest that psychedelic plants may have antimicrobial activity against pathogenic organisms, there has been no investigation of the effects on commensal bacteria and psychedelic compound exposure. With increasing interest in the roles of commensal gut ecology in health and disease, there has been more investigation into the psychoactive effects and complex host-organism interactions with bacterial colonies in the gastrointestinal tract. Microbiome bacterial populations appear to have play a significant role in a number of immune responses and have been found to influence the presence of self-antigens and AiD.
Gut bacteria have been discovered to possess the ability to manipulate neurotransmitter activity and are capable of producing serotonin, dopamine, acetylcholine, GABA, and more. Many of these neurotransmitters are derived from essential amino acids such as tryptophan, phenylalanine, and glutamine. Commensal gut bacteria possess highly conserved metabolic pathways for processing amino acids for a variety of uses. Many of the classic psychedelics are also biologically synthesized from tryptophan and share an analogous structure to serotonin and other endogenous tryptamine molecules. It is tempting to speculate that gut bacteria may produce these tryptamine metabolic products to use as signaling molecules meaning that bacteria are likely to not only produce these molecules, but also receive information from them as signal inputs. One study found that spore-forming endogenous bacteria species from
Turicibacteraceae, Clostridiales, Lachnospiraceae and Ruminococcaceae were enriched in abundance when 5-HT was orally administered to wild type rats suggesting that the secretion of 5-HT from gut bacteria and the host intestine may prompt an advantage towards thriving colonies and promote the bacteria’s own community membership in the mammalian intestine, rather than 5-HT simply being a metabolic byproduct. It is very possible that the psychedelic serotonin analogues psilocybin, DMT, and 5-MeO-DMT may interact with bacterial receptors such as those found in
Turicibacter sanguinis, a gut bacterium that expresses a neurotransmitter sodium symporter-related protein with sequence and structural homology to mammalian SERT. It is unclear if this SERT orthologue is solely responsible for the mechanism of transporting 5-HT into bacterial cells, but spore-forming gut bacteria do show the ability to transport 5-HT through some mechanism of action. If serotonergic psychedelic compounds were found to interact with these SERT-homologous bacterial proteins or other bacterial 5-HT uptake pathways in a way that alters the behavior of the bacterial colonies, this could have implications on gut microbe ecology and thus AiD disease outcome.
Whether there are direct pharmacological effects of psychedelics on bacteria is currently unknown, however the emotional and psychological benefits of psychedelics may indirectly alter microbial communities in the GI tract
via induced changes in vagal nerve tone, stress response, and enteric environment. Several studies have explored the effects of chronic stress and resulting conditions like PTSD on microbiome pattern associations. The psychological and neurological benefits from a psychedelic experience may create biochemical cascades in systemic physiology, specifically within the HPA axis and the enteric nervous system, which may downstream influence the ecology of microbial populations.
As mentioned previously, LPS is a cell wall component of Gram-negative bacteria. LPS is known to induce inflammation and is used in research as an inflammatory trigger in animal models to simulate Parkinson’s, Alzheimer’s, MDD and general inflammatory conditions. In patients with increased gut-permeability, LPS fragments can leak past the intestinal epithelial barrier and cause inflammation while in circulation. Models using LPS-induced inflammation were used in some of the previously noted human
invitro, and rat studies on the anti-inflammatory effects of psychedelics. This research may be particularly relevant to AiD as it suggests psychedelics may be helpful in mitigating inflammation caused by LPS due to increased intestinal permeability and chronic gut dysbiosis.
2.8. Mitigating the risks of psychedelic side-effects in therapeutic settings
Given the powerful psychoactive effects of psychedelic compounds, their use in medicine and psychiatry should not be taken lightly. Currently most psychedelic compounds are schedule I compounds meaning they have the highest categorical ranking of illegality in the Unites States with Schedule I stating that a substance has no acknowledged medical use, and is considered to have high-abuse potential.
Most psychological harms associated with the illegal use of psychedelics appear to be a result of poorly controlled environmental factors such as set and setting, as well as questionable sources of the drugs from the black market. In clinical administration of psychedelics, there is documented very low chance of psychological harm to properly screened patients provided with integrative support. One population study suggests that psychedelics do not appear to linked to long term mental health problems or suicidality. One 2010 analysis of psilocybin studies done between 1999 and 2008 looked at the experiences of 110 patients. Negative experiences were not common and seemed to be dose-dependent where higher doses of psilocybin were associated with higher rates of adverse reactions. All of the short-term adverse reactions were “successfully managed through interpersonal support” and did not require taking medications and seemed to have no lasting effects, based on follow-up interviews.
Hallucinogen Persisting Perception Disorder (HPPD) is a controversial neurocognitive condition where the patient reports persisting sensory effects after using a psychedelic substance. Also known as “flashbacks” the patient may experience brief re-occurrences/episodes of alterations in perception, mood, and/or consciousness, as previously experienced during a hallucinogenic intoxication. These symptoms can occur on a spectrum from occasional to near-constant. There is little conclusion about how common or debilitating these “flashback” experiences may be. Some studies suggest that they are less likely to occur in those who have taken a psychedelic in a clinical setting. Based on the phenomena existing in psychedelic-naïve patients and inconclusive data from studies that have examined the disorder, a causal relationship between persistent perceptual symptoms and use of psychedelics remains unproven.
Some precautions that may reduce harm to those receiving psychedelic medicine in clinical environments may include screening for a history of schizophrenia or psychosis, comfortable therapeutic set and settings that reduce the risk of panic or distress, interpersonal support provided by the clinicians, pharmaceutical-grade drugs, and low, subperceptual dosing, also known as micro-dosing.
3. Discussion and perspectives
There is vast opportunity to explore the effects of psychedelics on the immune system, in particular, autoimmunity. Systematic screening for various autoantibodies, inflammatory biomarkers, and for the expression of autoimmune-related genes in response to psychedelic treatments could provide intriguing observations and lead to more focused investigations.
The effects of psychedelic compounds on enteric bacterial behavior is of particular interest. Studies could be conducted examining the effects on bacterial growth and metabolism as a result of exposure to psychedelic compounds.
In vitro and
in vivo studies may hold many answers and even more questions about if and how serotonergic psychedelic compounds may be transported into bacterial cells and influence microbiome colonization and species composition. Studies could examine the effect of administration of psychedelics on enteric epithelial tissue integrity in the gut, or effects on LPS-induced cytotoxicity,
etc.
Although results from preclinical studies on the immunomodulatory and anti-inflammatory effects are promising, the field of psychedelic research in biomedicine is still in its infancy. Current hypotheses regarding signaling mechanisms and systemic immune effects of psychedelics are based on a very limited amount of factual data. Only until we better understand the genetic and pathophysiological bases for AiD can there be appropriately and rigorously designed human clinical studies that consider psychedelics as potential therapeutic agents.
While there is a need for deeper investigation, there appears to be enough evidence of direct and indirect effects from psychedelic compounds that may benefit those with AiD to merit further exploration on the topic. Given the complexity of factors that contribute to AiD, a multi-facetted approach may be appropriate to address the multiple features and causes of AiD simultaneously. Unlike many current conventional treatment methods, it appears that psychedelics may potentially offer an efficacious strategy for relieving and perhaps even resolving autoimmunity by targeting psychospiritual origins, maladaptive chronic stress responses, inflammatory pathways, immune modulation and enteric microbiome populations. The evidence presented in this paper provides support to the idea that there is untapped potential of exploring the use of psychedelics within this specific disease category. Given the limitation of efficacious treatment options for AiDs, and the physiological safety of psychedelic substances, it is likely a research topic worth pursuing. There is hope that the collection of this evidence may guide or inspire others to pioneer such studies. Overall, this direction of research is virtually unexplored and underdeveloped, providing a wealth of opportunities to discover novel applications for psychedelics in the field of immunology.
With a rise in the incidence of autoimmune diseases (AiD), health care providers continue to seek out more efficacious treatment approaches for the Ai…

www.sciencedirect.com




 www.lucid.news
www.lucid.news