paracelsius
Bluelighter
- Joined
- Mar 11, 2020
- Messages
- 197
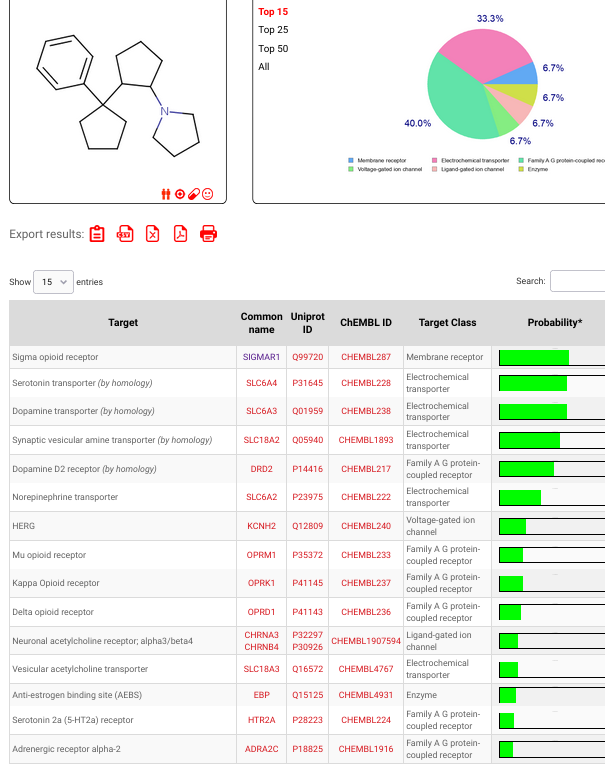
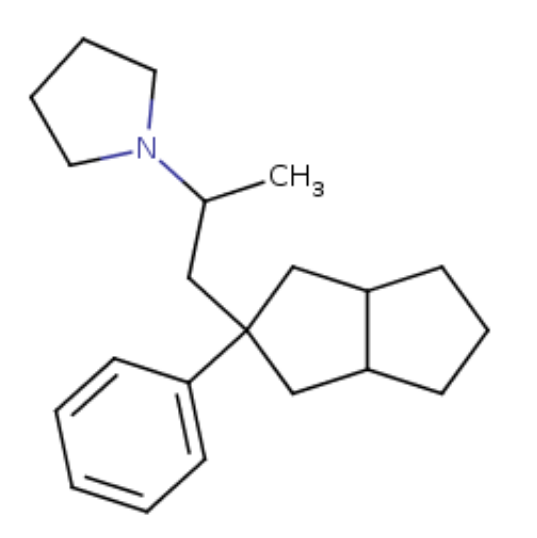
I was quite intrigued reading that sinovion patent about arylcycloalkyl-methylamines that are extremely potent triple SNDR uptake inhibitors. Not arylcycloalkylamines disso. Basically move the amine of arylcyclohexylamines up one carbon for example like this:
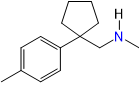
Now looking closely it looks like 4-methylmethcathinone (mephedrone, 4MMC):

a classic triple SNDRI and releaser with its carbonyl replaced by a cyclopentyl.
Now this cyclopentyl compound above is some 300x more potent at SERT DAT NET than meth but unlike meth or meph is straightforward cocaine-like substrate reuptake inhibitor (balanced) but not releaser. Now would it also be a NMDAr antagonist? (couldnt find any info on that).. My guess is probably not... way more easier to synth than the arycylcohexylamines in 2 steps from dirt cheap para-methyl-tolunitrile!! but then again it is a STILL a phenenethylamine distant cousin, or is it?!!
EDIT: I don't trust patent literature too much..sometimes they exaggerate but it looks like they did pretty extensive SAR on this class

Now looking closely it looks like 4-methylmethcathinone (mephedrone, 4MMC):

a classic triple SNDRI and releaser with its carbonyl replaced by a cyclopentyl.
Now this cyclopentyl compound above is some 300x more potent at SERT DAT NET than meth but unlike meth or meph is straightforward cocaine-like substrate reuptake inhibitor (balanced) but not releaser. Now would it also be a NMDAr antagonist? (couldnt find any info on that).. My guess is probably not... way more easier to synth than the arycylcohexylamines in 2 steps from dirt cheap para-methyl-tolunitrile!! but then again it is a STILL a phenenethylamine distant cousin, or is it?!!
EDIT: I don't trust patent literature too much..sometimes they exaggerate but it looks like they did pretty extensive SAR on this class
Last edited: