flying-potato
Greenlighter
- Joined
- Sep 4, 2015
- Messages
- 39
hello,
first, i know I'm a newbie and my English is not the best out there. My chemical knowledge is not the best too.
I studied chemical science a while ago so maybe i forget many stuff.
Few years ago i was a big fan of 4-MMC but it disappear. I see there was many attempt to find some chemical that
may have the same effect, but judging of the report no one really match.
After seeing many design i asked myself why nobody tried to begin with a 1-tetralone base.
https://en.wikipedia.org/wiki/1-Tetralone
So i reflected about it and designed 7-MMAT (7-Methyl-N-Methyl-2-Amino-1-Tetralone) and find a theoretical way to do the synthesis.
Is there any reason why this chemical might be a bad idea ?
Step 1: Methylation of 1-Tetralone to form 7-Methyl-1-Tetralone, must to care to not do over methylation.
Not reflected much about how to solve this problem.
Step 2: Bromination of the compound to form 7-Methyl-2-Bromo-1-Tetralone
This study find a way to do it on 1-Tetralone : http://www.rsc.org/suppdata/gc/b7/b707065a/b707065a.pdf
Step 3: Substitution of the bromine with Methylamine. This step have 2 problem, the first is the formation of
an imine group in the place of the ketone, this can be avoided or reversed with water. The second is the risk of
over akylation of the amine, but must to some way to avoid it.
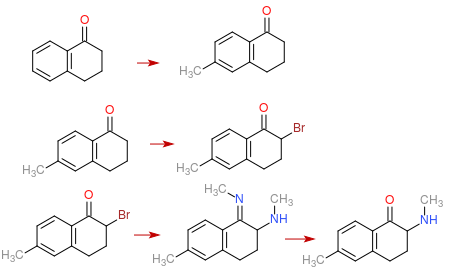
Is there something wrong somewhere ?
first, i know I'm a newbie and my English is not the best out there. My chemical knowledge is not the best too.
I studied chemical science a while ago so maybe i forget many stuff.
Few years ago i was a big fan of 4-MMC but it disappear. I see there was many attempt to find some chemical that
may have the same effect, but judging of the report no one really match.
After seeing many design i asked myself why nobody tried to begin with a 1-tetralone base.
https://en.wikipedia.org/wiki/1-Tetralone
So i reflected about it and designed 7-MMAT (7-Methyl-N-Methyl-2-Amino-1-Tetralone) and find a theoretical way to do the synthesis.
Is there any reason why this chemical might be a bad idea ?
Step 1: Methylation of 1-Tetralone to form 7-Methyl-1-Tetralone, must to care to not do over methylation.
Not reflected much about how to solve this problem.
Step 2: Bromination of the compound to form 7-Methyl-2-Bromo-1-Tetralone
This study find a way to do it on 1-Tetralone : http://www.rsc.org/suppdata/gc/b7/b707065a/b707065a.pdf
Step 3: Substitution of the bromine with Methylamine. This step have 2 problem, the first is the formation of
an imine group in the place of the ketone, this can be avoided or reversed with water. The second is the risk of
over akylation of the amine, but must to some way to avoid it.

Is there something wrong somewhere ?
