Hello everyone, please keep in mind that I'm a noob in the field, so forgive any mistake I may make.
I had a question regarding 5-HT2B receptor structure-activity relationships in molecules. The reason I was wondering what kind of characteristics a molecule needs to have in order to act as an agonist on 5-HT2B receptors is speculation about a particular research chemical, 2-FMA. I'll get to this soon enough.
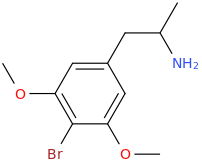
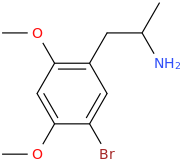
As you know, 5-HT2B agonism is associated with cardiac problems. I was looking at the Wikipedia page for the 5-HT2B receptor and it provides a list for its known agonists. Now, a thing I have noticed is that if the agonist has an amphetamine-like structure, then it basically always has to have something bound to the 3 or 4 position in the benzene ring, in order to show 5-HT2B agonistic activity.
For example:
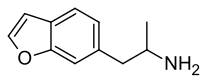
6-APB
MDMA
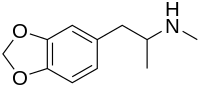
Norfenfluramine

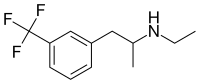
Fenfluramine
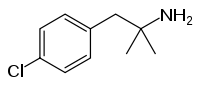
Chlorophentermine
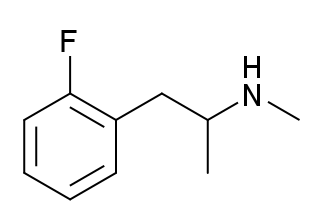
And the list goes on and on. As you can see, we basically always have something bound to the 3rd or 4th position in the benzene ring. Could this be required in order to display 5-HT2B agonism? And in that case, is it possible that 2-FMA (molecule shown below) wouldn't manifest it (fluorine bound to the 2nd position)?
I had a question regarding 5-HT2B receptor structure-activity relationships in molecules. The reason I was wondering what kind of characteristics a molecule needs to have in order to act as an agonist on 5-HT2B receptors is speculation about a particular research chemical, 2-FMA. I'll get to this soon enough.
As you know, 5-HT2B agonism is associated with cardiac problems. I was looking at the Wikipedia page for the 5-HT2B receptor and it provides a list for its known agonists. Now, a thing I have noticed is that if the agonist has an amphetamine-like structure, then it basically always has to have something bound to the 3 or 4 position in the benzene ring, in order to show 5-HT2B agonistic activity.
For example:
6-APB

MDMA

Norfenfluramine

Fenfluramine

Chlorophentermine

And the list goes on and on. As you can see, we basically always have something bound to the 3rd or 4th position in the benzene ring. Could this be required in order to display 5-HT2B agonism? And in that case, is it possible that 2-FMA (molecule shown below) wouldn't manifest it (fluorine bound to the 2nd position)?