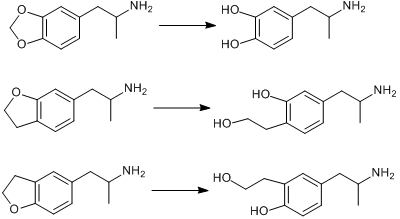
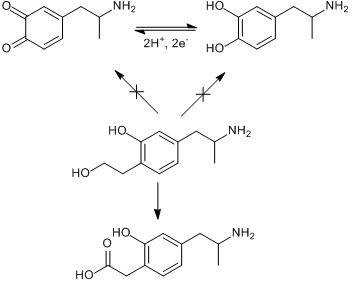
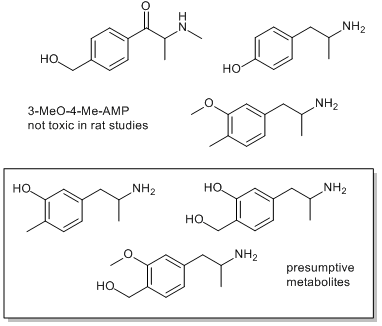
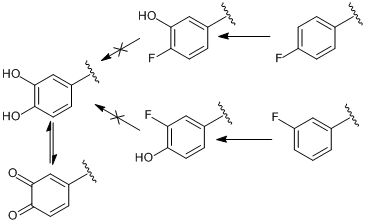
Does anybody have any information on the pharmacology of this chemical and the metabolism routes in takes into the body?
I'm wondering how similar to MDMA/MDA it is. I really can't find much information at all on the chemical, but it just felt so similar to MDA/MDMA that I'm wondering how similar it acts.
Thanks!
I'm wondering how similar to MDMA/MDA it is. I really can't find much information at all on the chemical, but it just felt so similar to MDA/MDMA that I'm wondering how similar it acts.
Thanks!