Drugs are a temporary solution to a long-lived problem.
Suicide is a permanent solution to temporary problem.
I've watched someone die as their kidneys failed. So I stay alive because should a severe head injury occur, At least I can give others the chance. Heart, lungs, kidneys, eyes and so forth. Suicide means an inquest so those organs help nobody.
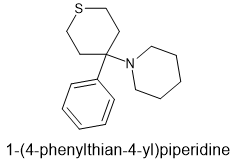
BTW Why are you moving the amine away from the aryl moiety? Note dizocilpine, the prototype NMDA antagonist or diphenidine. Their has to be an aryl just 1 methylene from the aryl. Identify the key moieties and consider their spatial relationship (including the N

.
The first effort was so close - just the LogP which, BTW, you could have brought down by adding a ketone. Where? Dunno.
1-(3-Methyl-4-thiophen-2-ylthian-4-yl)piperidine | C15H23NS2 | CID 15408084 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.

pubchem.ncbi.nlm.nih.gov
Follow the patents - voila, 78 NMDA antgonists/DRIs.