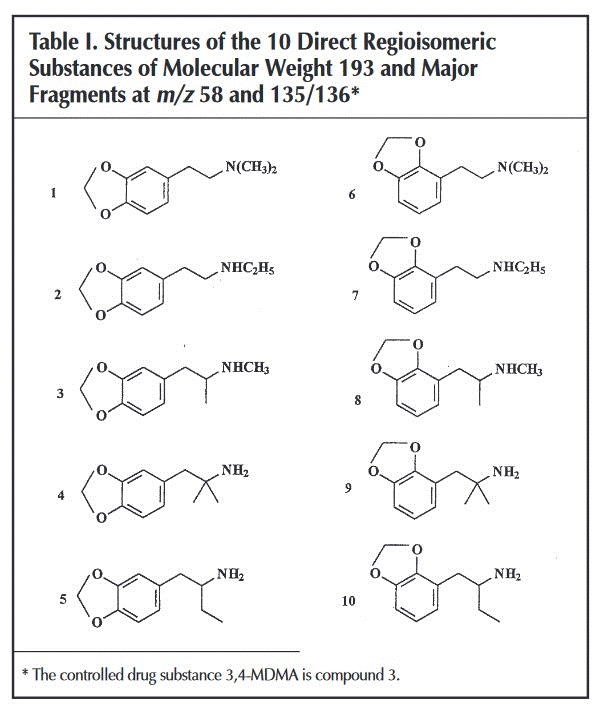
Not at all, GC can separate between substances with different molecular weights but can't for instance differentiate between a 2,3-whatever from a 3,4-another. In fact if you read the paper I posted in the previous page
https://pubs.acs.org/doi/pdf/10.1021/acs.analchem.0c00915, the amount of effort to differentiate between 2, 3, and 4MMC, and 2,3-mda/mdma with their relative 3,4-compund is admirable, they even had to customise and modify their instrumentation to be able to achieve that. It is not simple when you have to compare the same compound rearranged in a different way, the game here is at very high levels.
That's why this thread after 4 years is still here.
This paragraph is particularly interesting:
MDMA-Type Isomer Differentiation with Infrared IonSpectroscopy.
Another, different, class of isomeric com-pounds that have a bicyclic ring structure connected at
different positions, the MDMA- and MDA-type drugs, were also investigated. The 3,4-isomers of both MDMA (known as“ecstasy”) and MDA are frequently occurring stimulants that
are controlled substances in The Netherlands. However, their 2,3-isomers are both NPS that are not controlled and are
nearly indistinguishable from their corresponding 3,4-isomers on the basis of GC-MS, being the method of choice in most
forensic laboratories.11 Using IRIS, however, clearly distinctive IR spectra for each isomer could be generated which enables
simple identification of these NPS from their controlledcounterparts, as presented inFigure 3(see Figure S3for a comparison to computationally predicted IR spectra) (Note. NPS=Non Prohibited Substances).
This is why I strongly tend to believe that the precursor used is 2,3-MDP2P glycidate, it is legal, it goes thru customs and in the event of LEO operations there still a hard time for the law to prove that it is a illegal precursor/substance, furthermore it is practically indistinguishable from its more active relative MDMA on a street an up levels.
Exactly! why you want to add such a substance with extreme dosage dangers, cost, and for what? to make your own product half as potent so you can sell more??? nah I don't buy it at all sounds like a street myth.
Still for the quantities involved in the hypothetical contaminant if the active is in nanograms it won't crystallize along with the main product, we're talking about that .001% of cut, it is fervid imagination IMO. It will stay in that part of the product discarded when going to 75 to high 90's as you said.
Also re-crystallization is a standard procedure in refining pharmaceuticals.
Again.. wrong... I'm in my 50's, I used MDMA from 1995 to around 2002, several times a year, commercial tabs in the 90's and pure mdma later, produced locally by a small lab.
I can explain with a pic the differences from Magic and Meh
Polymorphism affects substances solubility only in very particular cases, and not MDMA which is always soluble in water.
Unfortunately if my hypothesis is correct we'll never see again mass produced Magic MDMA.
(edit: typo's)