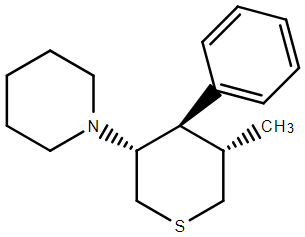
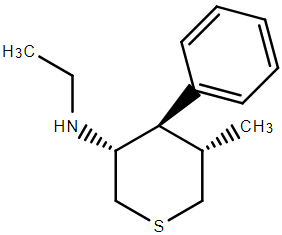
Image k3 hosted in ImgBB

ibb.co
I THINK I have the drawn correct isomer. The problem was that while 4 of the inactives were simple to remove, the last 3 unwanted required kinetic resolution. Of course, the stuff was an order of magnitude more potent that PCP so as long as the inactives were non-toxic, they could be left in the mix. What the paper did not cover was the N-monosubstituted analogues. In such a case. maybe some more interesting analogues would be possible.
Image k4 hosted in ImgBB

ibb.co
That said, the chiral bulk of the piperidine may have altered the orientation of other moieties.
People forget that K/PCP are only pleasurable because they also act as DRIs and it remains to be seen if either of the above have such activity. I've said it once and I will say it again, the very simplest NDMA/DRI ligand is isophenidine. The 2-phenyl is a bioisostere of an ethane so the whole compound is a bioisostere of N-Isopropyl-1-phenyl-2-pentanamine. Well, N-methyl-1-phenyl-2-pentamine turned up as an RC (a DRI) some time ago. Synchronicity has it that the (R) isomer is an SRI while the (S) isomer is an NMDA antagonist. In short, the synthesis yields 2 different actives that act in concert to produce an ASC very similar to K. Nothing is wasted.
A cohort of 78 people tried isophenidine and 58 of them expressed the opinion that while somewhat different to K, it was superior. Double blinding with K showed that many people could not differentiate the two. I was tempted to replace the 2-phenyl with a 2-p-tolyl moiety but sadly the precursor was not available commercially and custom synthesis cost a fortune. In any case, isophenidine proved very popular and the $/Kg is measured in the hundreds (at scale). Further more, any bench-scale chemist can make it.
The synthesis is amazingly simple. 1,2-diphenylethanone (207-193-2) is dirt cheap and since isopropylamine is a primary amine, an imine rather than an enamine was formed during the dehydration. (amine+ketone-->imine + H2O), so the reaction proceeds much more readily. The trick is to use isopropylamine hydrochloride (15572-56-2) and once it has dissolved in methanol (the only solvent needed), careful addition of potassium hydroxide (75-31-0) will freebase the IPA.HCl AND abstracts the water formed by the amine--->imine step. Now the imine is MUCH less soluble that the amine in methanol so that the intermediate imine is crashed out forming a separate layer. The use of a vessel with a drain at the bottom, the raw imine can easily be decanted into a second vessel filled with a larger methanol to ensure complete dissolution. NaBH4 is not affected by alkali conditions and so the addition of NaBH4 to the imine reduces imine to amine in highy yield and purity.
All told, this is MUCH simpler chemistry than the average methamphetamine synthesis and since the solvents are relatively cheap, available & non-toxic, excess methanol (CH3OH) in the final product can be easily removed by careful heating of the raw product in a warm environment free of ignition sources.
At this stage the freebase of the product is formed and the ONLY minor issue is that the product isn't too soluble as it's hydrogen chloride addition salt (nasty to snort). Much research was undertaken and the sulfate salt proved the most soluble although the phosphate proved a reasonable option. IF I intended to infuse the product, I would go for the sulfate.
To conclude - two simple reactions that yield materials easy to manipulate. Considering the known toxicity of K makes this compound useful in HR policy. Of course, toxicity of this compound would need to be examined.
In conclusion - isophenidine proves to be an excellent alternative to ketamine (or index methoxetamine) given it's similarity in action, synthetically simple production and production cost about 10% that of ketamine and not needing the specialist production facilities required of ketamine derivatives.
Of course, science has developed much more potent ligands but their synthetic complexity makes them unattractive tarkets.