paracelsius
Bluelighter
- Joined
- Mar 11, 2020
- Messages
- 197
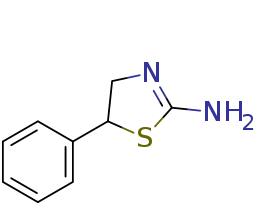
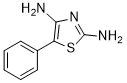
It is a sulfur homolog (5-phenyl-2-amino-1,3-thiazoline) of Aminorex (5-phenyl-2-amino-1,3-oxazoline):
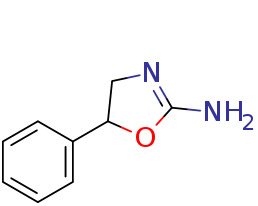
with the oxazoline ring of Aminorex replaced by a thiazoline. This compound has been claimed as Nootropic Anorexic and Sexual Aphrodisiacs safer than the Aryloxazolines (AR, 4-MAR, 4,4'-DMAR, pemoline, cyclazodone..etc) stimulants. Unlike aryloxazolines, it is a selective norepinephrine-dopamine reuptake inhibitor (NDRI) with low serotonergic SERT activity (about 80x selectivity for DAT~NET v. SERT).
Now my question is: what would make it safer? For one, less serotonergic will surely reduce the risk of (possible) toxic serotonin syndrome of large doses of non-selective SDNRI/A such as AR, 4-MAR and especially 4,4'-DMAR. But serotonergic toxicity of ARs is due to massive serotonin release (not uptake inhibition) AND the fact AR, 4-MAR, especially 4,4'-DMAR have no TAAR agonist activity like MDMA to feedback regulate levels of serotonin released.
So besides being monoamines uptake inhibitors, ARs are also potent monoamines releasers (EC50~8-20nM, 10x more potent than MDMA on avg!) with no TAAR agonist activity like MDMA and related entactogens that are also TAAR agonists in addition to being monoamines releasers. Aryloxazolines have a rather unique profile: on avg 10x more potent for DAT/NET v SERT for uptake inhibition and the exact opposite selectivity for release (10x more potent for SERT release v DA~NE!).
Now, wouldn't that compound also be a releaser?? the problem with ARs serotonin syndrome risks and sexual side-effects (stim "limp d!ck" in males) is due to serotonin release unregulated by TAAR agonism, not necessarily uptake inhibition!. I have yet to see whether this compound class (Arylthiazolines) are also releasers and if yes, what is their selectivity profile? Sure, they will be safer than Aryloxazolines if they are also selective DA/NE releasers with no or less serotonin release, in case they do have monoamines releasing properties like the Aryloxazolines. Else, they'll certainly make safer nootropic anorexic stimulants and...potent sexual aphrodisiacs devoid of sexual side-effects ("stim "limp d!ck") much like other selective NDRI/As like MDPV and other pyros.
edit: I do not endorse any NPS but for harm reduction sake, I thought this will be interesting to post on NPD.