Sturnam
Bluelighter
- Joined
- Aug 12, 2008
- Messages
- 738
nabollocks said:Buprenorphine would do the same thing... that does not mean that it has no place in w/d.
This is really interesting, and I cannot wait to get to the bottom of it.
Well, wouldn't that fact that it precipitates w/d and fails to suppress w/d symptoms mean that it is useless for treatment?
And as far as the CD50, it seems like its the same across the board for several different animals. Any ideas why?
Convulsions were observed in almost all species of animals including the skate, frog, sparrow, pigeon, mouse, guinea pig, rabbit, cat and dog. In the rhesus monkey, transient tremors, restlessness, hyper-irritability and convulsions were observed. The convulsive doses were around 20 mg/kg s.c. in mice, rabbits, cats, dogs and rhesus monkeys.
Also, does anyone have an idea what mediates the convulsant effects of thebaine? this article says that it's not anything you can develop tolerance to, which seems like it would not be associated with the opioid receptors.
In an attempt to produce tolerance to the convulsant effect of thebaine in rhesus monkeys, Yanagita and Miyasato repeatedly administered 2 mg/kg of thebaine 6 to 24 times daily for 6 weeks or longer to 4 monkeys by programmed injection through intravenous in-swelling catheters. Every two weeks the monkeys were challenged with 4 mg/kg of thebaine which is the convulsant dose. No evidence for the development of tolerance to the convulsant effect of thebaine was obtained.

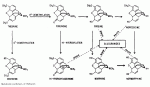

 ) GLyRs in spinal neurones.
) GLyRs in spinal neurones.