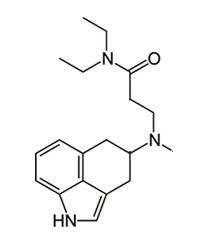
^ Interesting, be careful and keep us posted pls

The duration seems hard to predict and I'd 'hope for the best prepare for the worst', but if I would take a
wild guess I think maybe something like 2 hours shorter than LSD? On account of the primary metabolism not really happening at positions that are different (the 4th ring), but instead just dealkylation and 2-oxo-3-hydroxy oxidation of the indole nucleus, all of which would still be expected to happen with NDTDI. But maybe a bit easier if enzymes gain more steric access to the structure, hence the guess that it might be a little shorter. [Or would it be a LOT shorter because MALT / 5-MeO-MALT are as well?]
Mostly saying that as a gamble to see if it turns out to be a close estimation. Like I said: clear your schedule to be sure.