-
Psychedelic Drugs Welcome Guest
PD's Best Threads Index Social ThreadSupport Bluelight Psychedelic Beginner's FAQ
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
☛ Official ☚ The Small & Handy NDTDI Thread
- Thread starter bjznoviskey
- Start date
Bigazznugz
Bluelighter
- Joined
- Feb 11, 2013
- Messages
- 887
Nope its still coming. The yields of this are very small and i think they are only making 30g batches at a time. Seems like a very tricky synth along the lines of making lsd.
My buddy has a gram of this on the way this week.
Once he gets it i should have a sample coming my way.
I think they just took that off the site to add MORE insane fentanyl anaouges.
Tetrohydrofuran fentanly? Jesus they are killing the reseach chemical community by doing so. Its just gonna lead to blanket bans sigh.
But yes nditi is coming. Eventually.
My buddy has a gram of this on the way this week.
Once he gets it i should have a sample coming my way.
I think they just took that off the site to add MORE insane fentanyl anaouges.
Tetrohydrofuran fentanly? Jesus they are killing the reseach chemical community by doing so. Its just gonna lead to blanket bans sigh.
But yes nditi is coming. Eventually.
kidklmx
Bluelighter
- Joined
- May 16, 2012
- Messages
- 1,311
Well I'm intrigued. Be sure to let us know about your trials. Given the novelty make sure you have zero to no tolerance, do an allergy test and from there titrate upwards slowly, etc. I hope it's any good and not something like Escaline where, yes, it feels like something that acts on the right serotonin receptors, but never gets really psychedelic.
Bigazznugz
Bluelighter
- Joined
- Feb 11, 2013
- Messages
- 887
Yeah i will most deffenity be very cautious with this one but i acutally have some hope for these NDTDI/trycyclic typtamined series, a also heard that there will be another chemical with a very similar stucture is being made now also. So i dont think they are going through so much trouble and i guess also apparently very high precursor/production costs to pull it prior to being released.
Hopefully since it seems that these were to difficult for most chinese labs to produce chemicals that are constrained simplifed versions of lsd, so mabey one day soon i can see this
https://en.m.wikipedia.org/wiki/RU-28306
It has always looked like it could have very high potential if made.
Lastly i would like to know if this chemical or anything along the lines of trycyclic tryptamines and or simplified non constrained lysergamines are even coverd by the U.S.A analogue act?
Hopefully since it seems that these were to difficult for most chinese labs to produce chemicals that are constrained simplifed versions of lsd, so mabey one day soon i can see this
https://en.m.wikipedia.org/wiki/RU-28306
It has always looked like it could have very high potential if made.
Lastly i would like to know if this chemical or anything along the lines of trycyclic tryptamines and or simplified non constrained lysergamines are even coverd by the U.S.A analogue act?
Last edited:
perpetualdawn
Bluelighter
- Joined
- Nov 20, 2013
- Messages
- 2,920
I've thought about ru-28306 too, very intriguing chemical, structurally. Would be really interesting if that got made. It's the perfect intersect between trytamine, phemethylamine and lysergamide.
Bigazznugz
Bluelighter
- Joined
- Feb 11, 2013
- Messages
- 887
Where did you hear that. I thought the same thing.IF someone has a sample, please contribute towards research and do quick reagent testing on it. It appears that at least one vendor is selling NDTDI as 1-butyl-LSD
uncle_bud
Bluelighter
- Joined
- May 29, 2012
- Messages
- 95
Well... The sample provided to me as 1-butyr-LSD was sent to Energy Control, Spain; for verification and they couldn't confirm it, so they are in the process of repeating the analysis. And one of my friend working there was suggested that it might be NDTDI since it's currently circulating and that would also explain why there was an immediate purple with ehrlich's reagent. (open ring).
Maybe you were the one
Maybe you were the one
perpetualdawn
Bluelighter
- Joined
- Nov 20, 2013
- Messages
- 2,920
@bigazznugz did you ever test this NDTDI out?
Phenpsycho
Bluelighter
- Joined
- Nov 28, 2015
- Messages
- 122
Really interesting looking compound! Looking forward to reading the TR on this one as well.
Bigazznugz
Bluelighter
- Joined
- Feb 11, 2013
- Messages
- 887
No the vendor nvever recievird the succinate. So he is thinking about asking for 20gsof the newdmt analog ru 28870 or whatever its called.
IF someone has a sample, please contribute towards research and do quick reagent testing on it. It appears that at least one vendor is selling NDTDI as 1-butyl-LSD
How is that possible?
If NDTDI is active dosage around 5mg and 1-butyl-LSD is way less.
Do the vendor sell mixed NDTDI?
Solipsis
Bluelight Crew
- Joined
- Mar 12, 2007
- Messages
- 15,509
How is that possible?
If NDTDI is active dosage around 5mg and 1-butyl-LSD is way less.
Do the vendor sell mixed NDTDI?
Certainly not NDTDI mixed with 1-butyl-LSD because it is unlikely that he would possess both and there is no motive.
If you mean one of them was cut meaning mixed with something inactive, then that is possible but it would not be suspected or detected without testing / analysis because it would be cut to make the supposed potency about the same (not very likely, the potency is very new and therefore uncertain data for both). Right now it is suspected to be misadvertised exactly because it is NOT the expected potency. A drug being much more or much less potent than it is expected to be is always suspicious, you need a reference of someone saying what the potency is supposed to be of course, otherwise you are likely to accept whatever the potency turns out to be.
Anyway, you could only cut 1-butyl-LSD at a 1:5 ratio and sell it as NDTDI but you cannot make NDTDI more potent than it is 100% to sell it as 1-butyl-LSD so that is an impossible scenario.
To be clear: it may not be so simple to differentiate between such substances, Ehrlich's may appear positive for both, but for 1-acyl lysergamides there is supposed to be a delay in the color reaction. I guess more sophisticated analysis is needed.
What else do we really know about these substances that could tell us how to identify them reasonably?
Bigazznugz
Bluelighter
- Joined
- Feb 11, 2013
- Messages
- 887
Nothing ndtdi from what i have heard from the dude who runs the legal lsyergamine operation that ndtdi is a complete fail. Or not remotely worth the money it costs.
I have heard from them that it does look very similar to ndtdi. But it coukd literally be anything.
I suspect its ndtdi but one could also be ru 28306. I say its most likley ndtdi because of the IMMEDIATE reaction to echlich. I cant comment on how ru 28306 reacts.
I can ask the one person i know who has it to preform a echlich test but op has not checked back in and i doubt ndtdi will make it outside of china and the very few vendors who hot scewed for buying it.
I have heard from them that it does look very similar to ndtdi. But it coukd literally be anything.
I suspect its ndtdi but one could also be ru 28306. I say its most likley ndtdi because of the IMMEDIATE reaction to echlich. I cant comment on how ru 28306 reacts.
I can ask the one person i know who has it to preform a echlich test but op has not checked back in and i doubt ndtdi will make it outside of china and the very few vendors who hot scewed for buying it.
I cant comment on how ru 28306 reacts.
Ru28306 should almost certainly react with Ehrlich's since it's an indole... after all, it's just DMT with a carbon between the 4-position on the aromatic ring and the alpha-position on the ethyl chain.
Shame about NDTDI though. It might have been an interesting lead compound for a series of psychs if someone like Shulgin or Nichols had investigated different analogs with alkyl groups of different sizes. Like... look at how Phenethylamines decrease in potency with a simple alkyl on the nitrogen, yet slap on a Methoxybenzyl and you suddenly have an NBOMe that's 20 times as potent (or conversely how 2C-C is one of the milder 2C's, whereas 25C is possibly the strongest NBOMe in terms of dosage).
Solipsis
Bluelight Crew
- Joined
- Mar 12, 2007
- Messages
- 15,509
All bets are off with SAR, it's no good expecting something like 25C to be similar to 2C-C with a reasonably big difference like the n-benzyl group, which can force the orientation in 5-HT2A to be quite different. The astonishing thing is that despite certain similarities in structure between say PEAs and tryptamines, I think some actually seem to bind flipped in orientation compared to others.
Not sure how much similarity in orientation there is between lysergamides and NBX PEAs. Also I wonder if any similarities like between the 4-chloro PEAs come from activity at receptors other than 5-HT2A where the activity is less altered.
NDTDI type stuff, RU-28306 or the NDEPAs are different in that they are constrained or simplified versions of existing drugs, mainly the lysergamides or a bit the tryptamines. Varying alkyl sizes, attaching a group that changes a lot of properties or simplifying / constraining a structure can all have results that I think often can't be compared, the approaches as to why making the modifications are expected to increase binding / activity are not the same.
I think indoles react with Ehrlich's unless the reaction that apparently makes some colored conjugated not-quite-symmetrical dimer-ish compounds is obscured. Delay of reaction with 1-acyls might be a sign of that? Usually indoles or lysergamides are not hindered on that side though. Maybe you also wouldn't get a color reaction if there are changes made internally in the indole nucleus that would prevent conjugated species to form? Unfortunately I forgot how similar or not such compounds are to something like indigo, it was really not easy to find info on Ehrlich's complexes.
What does "it looks similar to NDTDI to them" mean? How did they look at it?
Seems like there is a lot of mystery and suspicious surrounding these few compounds, but some of the suspicions are impossible. Speculation can be interesting but pure guesswork or suspicion about the ID seems pointless. It's best just waiting on analysis.
Not sure how much similarity in orientation there is between lysergamides and NBX PEAs. Also I wonder if any similarities like between the 4-chloro PEAs come from activity at receptors other than 5-HT2A where the activity is less altered.
NDTDI type stuff, RU-28306 or the NDEPAs are different in that they are constrained or simplified versions of existing drugs, mainly the lysergamides or a bit the tryptamines. Varying alkyl sizes, attaching a group that changes a lot of properties or simplifying / constraining a structure can all have results that I think often can't be compared, the approaches as to why making the modifications are expected to increase binding / activity are not the same.
I think indoles react with Ehrlich's unless the reaction that apparently makes some colored conjugated not-quite-symmetrical dimer-ish compounds is obscured. Delay of reaction with 1-acyls might be a sign of that? Usually indoles or lysergamides are not hindered on that side though. Maybe you also wouldn't get a color reaction if there are changes made internally in the indole nucleus that would prevent conjugated species to form? Unfortunately I forgot how similar or not such compounds are to something like indigo, it was really not easy to find info on Ehrlich's complexes.
What does "it looks similar to NDTDI to them" mean? How did they look at it?
Seems like there is a lot of mystery and suspicious surrounding these few compounds, but some of the suspicions are impossible. Speculation can be interesting but pure guesswork or suspicion about the ID seems pointless. It's best just waiting on analysis.
Solipsis
Bluelight Crew
- Joined
- Mar 12, 2007
- Messages
- 15,509
Oh sweet, with the carbenium keyword from wiki I was able to find it:
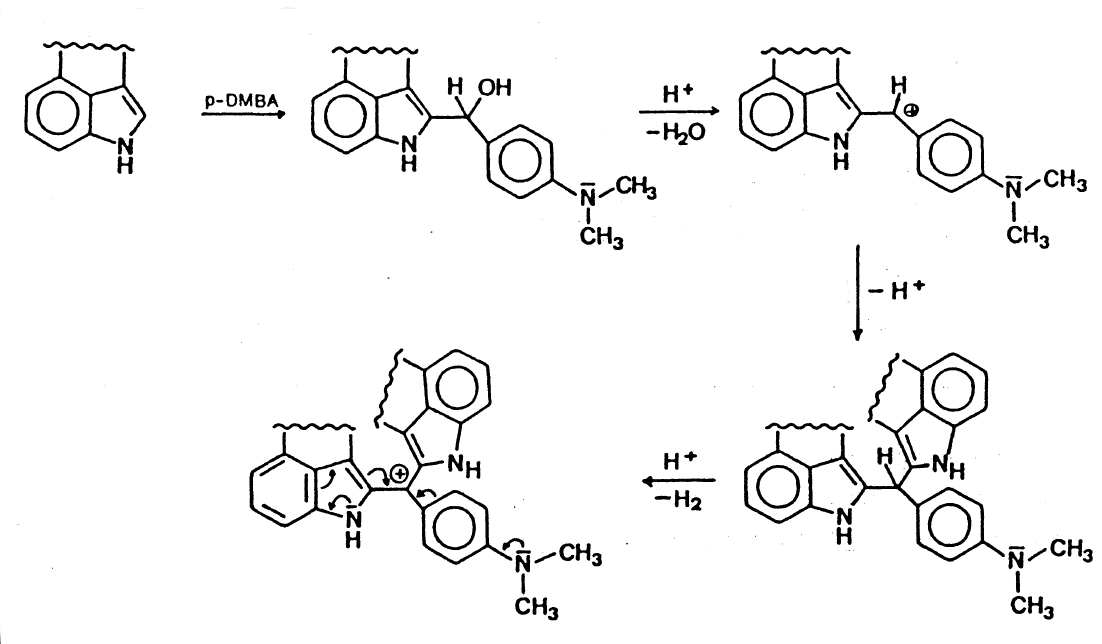
So yes it does demonstrate how indoles are in a semi-unique position for the reaction and disturbing the conjugation could prevent it. At least as valid a possibility as steric hindrance (not sure if it's necessarily hindered enough) seems to be that an 1-acyl makes the amine more basic which could be a problem for the conjugation. A much smaller fraction may turn into the quaternary amine so I think that makes the intermediate less favored.
I should have TMT or 5-MeO-TMT somewhere, if I access it again I could perhaps some day do an Ehrlich's which should not be positive due to the 2-methyl.
NSFW:

So yes it does demonstrate how indoles are in a semi-unique position for the reaction and disturbing the conjugation could prevent it. At least as valid a possibility as steric hindrance (not sure if it's necessarily hindered enough) seems to be that an 1-acyl makes the amine more basic which could be a problem for the conjugation. A much smaller fraction may turn into the quaternary amine so I think that makes the intermediate less favored.
I should have TMT or 5-MeO-TMT somewhere, if I access it again I could perhaps some day do an Ehrlich's which should not be positive due to the 2-methyl.
