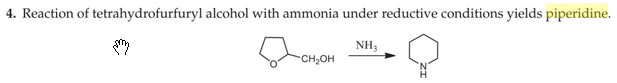
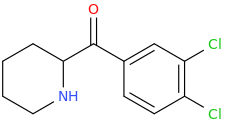
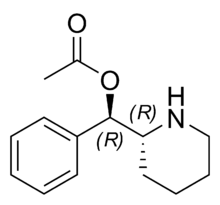
journal dump
Bis(4-hydroxy-N-isopropyl-N-methyltryptammonium) fumarate: a new crystalline form of miprocin
Andrew R. Chadeayne,a* Duyen N. K. Pham,b James A. Golenb and David R. Mankeb
The title compound, bis(4-hydroxy-N-isopropyl-N-methyltryptammonium) (4-HO-MiPT) fumarate (systematic name: bis{[2-(4-hydroxy-1H-indol-3-yl)ethyl](methyl)propan-2-ylazanium} but-2-enedioate), 2C14H21N2O+·C4H2O42−, has a singly protonated tryptammonium cation and one half of a fumarate dianion in the asymmetric unit. The tryptammonium and fumarate ions are held together in one-dimensional chains by N—H⋯O and O—H⋯O hydrogen bonds. [...]
Terpenoids Commonly Found in Cannabis sativa Do Not Modulate the Actions of Phytocannabinoids or Endocannabinoids on TRPA1 and TRPV1 Channels
Marika Heblinski, Marina Santiago, Charlotte Fletcher, Jordyn Stuart, Mark Connor, Iain S. McGregor, and Jonathon C. Arnold
Published Online:9 Mar 2020
Results: α-pinene, β-pinene, β-caryophyllene, linalool, limonene, β-myrcene or α-humulene did not affect [Ca]i in hTRPA1 and hTRPV1 overexpressing cells. Cinnamaldehyde (CA), Δ9-THC, and 2-arachidonoylglycerol (2-AG) activated TRPA1 receptors with high efficacy and similar potency (EC50s of ∼10 μM). Capsaicin and anandamide (AEA) activated TRPV1 receptors with an EC50 of 61 nM and 4.3 μM, respectively, but TRPV1 showed no response to Δ9-THC, cannabidiol, and other minor cannabinoids. Terpenoids did not significantly affect the responses of TRPA1 and TRPV1 receptors to submaximal and maximal concentrations of CA and Δ9-THC or the endocannabinoids AEA and 2-AG.
Discussion: We could not find any evidence that the terpenoids tested here activate TRPA1 and TRPV1 channels or modulate their activation by Δ9-THC and other agonists, including endocannabinoids.
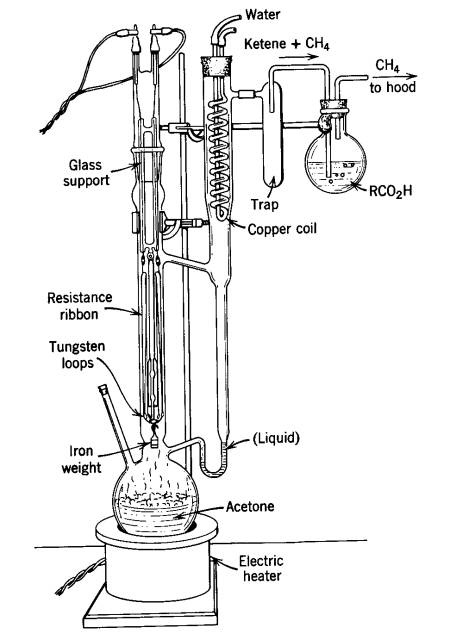
Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate
Dan Wu and View ORCID ProfileDonal F. O’Shea
PNAS March 24, 2020 117 (12) 6349-6355; first published March 10, 2020
Abstract
A combined analytical, theoretical, and experimental study has shown that the vaping of vitamin E acetate has the potential to produce exceptionally toxic ketene gas, which may be a contributing factor to the upsurge in pulmonary injuries associated with using e-cigarette/vaping products. Additionally, the pyrolysis of vitamin E acetate also produces carcinogen alkenes and benzene for which the negative long-term medical effects are well recognized. As temperatures reached in vaping devices can be equivalent to a laboratory pyrolysis apparatus, the potential for unexpected chemistries to take place on individual components within a vape mixture is high. Educational programs to inform of the danger are now required, as public perception has grown that vaping is not harmful.
Br J Anaesth. 2018 Jul;121(1):260-269. doi: 10.1016/j.bja.2018.03.014. Epub 2018 May 10.
Dreaming and awareness during dexmedetomidine- and propofol-induced unresponsiveness.
Radek L1, Kallionpää RE2, Karvonen M3, Scheinin A4, Maksimow A5, Långsjö J6, Kaisti K7, Vahlberg T8, Revonsuo A9, Scheinin H10, Valli K11.
BACKGROUND:
Experiences during anaesthetic-induced unresponsiveness have previously been investigated by interviews after recovery. To explore whether experiences occur during drug administration, we interviewed participants during target-controlled infusion (TCI) of dexmedetomidine or propofol and after recovery.
METHODS:
Healthy participants received dexmedetomidine (n=23) or propofol (n=24) in stepwise increments until loss of responsiveness (LOR1). During TCI we attempted to arouse them for interview (return of responsiveness, ROR1). After the interview, if unresponsiveness ensued with the same dose (LOR2), the procedure was repeated (ROR2). Finally, the concentration was increased 1.5-fold to achieve presumable loss of consciousness (LOC), infusion terminated, and the participants interviewed upon recovery (ROR3). An emotional sound stimulus was presented during LORs and LOC, and memory for stimuli was assessed with recognition task after recovery. Interview transcripts were content analysed.
RESULTS:
Of participants receiving dexmedetomidine, 18/23 were arousable from LOR1 and LOR2. Of participants receiving propofol, 10/24 were arousable from LOR1 and two of four were arousable from LOR2. Of 93 interviews performed, 84% included experiences from periods of unresponsiveness (dexmedetomidine 90%, propofol 74%). Internally generated experiences (dreaming) were present in 86% of reports from unresponsive periods, while externally generated experiences (awareness) were rare and linked to brief arousals. No within drug differences in the prevalence or content of experiences during infusion vs after recovery were observed, but participants receiving dexmedetomidine reported dreaming and awareness more often. Participants receiving dexmedetomidine recognised the emotional sounds better than participants receiving propofol (42% vs 15%), but none reported references to sounds spontaneously.
CONCLUSION:
Anaesthetic-induced unresponsiveness does not induce unconsciousness or necessarily even disconnectedness.