27 OCTOBER 2020
Sara Reardon
A handful of experiments are raising questions about whether clumps of cells and disembodied brains could be sentient, and how scientists would know if they were.
Illustration by Fabio Buonocore
In Alysson Muotri’s laboratory, hundreds of miniature human brains, the size of sesame seeds, float in Petri dishes, sparking with electrical activity.
These tiny structures, known as brain organoids, are grown from human stem cells and have become a familiar fixture in many labs that study the properties of the brain. Muotri, a neuroscientist at the University of California, San Diego (UCSD), has found some unusual ways to deploy his. He has connected organoids to walking robots, modified their genomes with Neanderthal genes, launched them into orbit aboard the International Space Station, and used them as models to develop more human-like artificial-intelligence systems. Like many scientists, Muotri has temporarily pivoted to studying COVID-19, using brain organoids to test how drugs perform against the SARS-CoV-2 coronavirus.
But one experiment has drawn more scrutiny than the others. In August 2019, Muotri’s group published a paper in Cell Stem Cell reporting the creation of human brain organoids that produced coordinated waves of activity, resembling those seen in premature babies. The waves continued for months before the team shut the experiment down.
This type of brain-wide, coordinated electrical activity is one of the properties of a conscious brain. The team’s finding led ethicists and scientists to raise a host of moral and philosophical questions about whether organoids should be allowed to reach this level of advanced development, whether ‘conscious’ organoids might be entitled to special treatment and rights not afforded to other clumps of cells and the possibility that consciousness could be created from scratch.
The idea of bodiless, self-aware brains was already on the minds of many neuroscientists and bioethicists. Just a few months earlier, a team at Yale University in New Haven, Connecticut, announced that it had at least partially restored life to the brains of pigs that had been killed hours earlier. By removing the brains from the pigs’ skulls and infusing them with a chemical cocktail, the researchers revived the neurons’ cellular functions and their ability to transmit electrical signals.
Other experiments, such as efforts to add human neurons to mouse brains, are raising questions, with some scientists and ethicists arguing that these experiments should not be allowed.
The studies have set the stage for a debate between those who want to avoid the creation of consciousness and those who see complex organoids as a means to study devastating human diseases. Muotri and many other neuroscientists think that human brain organoids could be the key to understanding uniquely human conditions such as autism and schizophrenia, which are impossible to study in detail in mouse models. To achieve this goal, Muotri says, he and others might need to deliberately create consciousness.
Researchers are now calling for a set of guidelines, similar to those used in animal research, to guide the humane use of brain organoids and other experiments that could achieve consciousness. In June, the US National Academies of Sciences, Engineering, and Medicine began a study with the aim of outlining the potential legal and ethical issues associated with brain organoids and human–animal chimaeras.
The concerns over lab-grown brains have also highlighted a blind spot: neuroscientists have no agreed way to define and measure consciousness. Without a working definition, ethicists worry that it will be impossible to stop an experiment before it crosses a line.
The current crop of experiments could force the issue. If scientists become convinced that an organoid has gained consciousness, they might need to hurry up and agree on a theory of how that happened, says Anil Seth, a cognitive neuroscientist at the University of Sussex near Brighton, UK. But, he says, if one person’s favoured theory deems the organoid conscious whereas another’s doesn’t, any confidence that consciousness has been attained vanishes. “Confidence largely depends on what theory we believe in. It’s a circularity.”
Sentient states
Creating a conscious system might be a whole lot easier than defining it. Researchers and clinicians define consciousness in many different ways for various purposes, but it is hard to synthesize them into one neat operational definition that could be used to decide on the status of a lab-grown brain.
Physicians generally assess the level of consciousness in patients in a vegetative state on the basis of whether the person blinks or flinches in response to pain or other stimuli. Using electroencephalogram (EEG) readings, for instance, researchers can also measure how the brain responds when it is zapped with an electrical pulse. A conscious brain will display much more complex, unpredictable electrical activity than one that is unconscious, which responds with simple, regular patterns.
But such tests might not adequately probe whether a person lacks consciousness. In brain-imaging studies of people who are in a coma or vegetative state, scientists have shown that unresponsive individuals can display some brain activity reminiscent of consciousness — such as activity in motor areas when asked to think about walking.
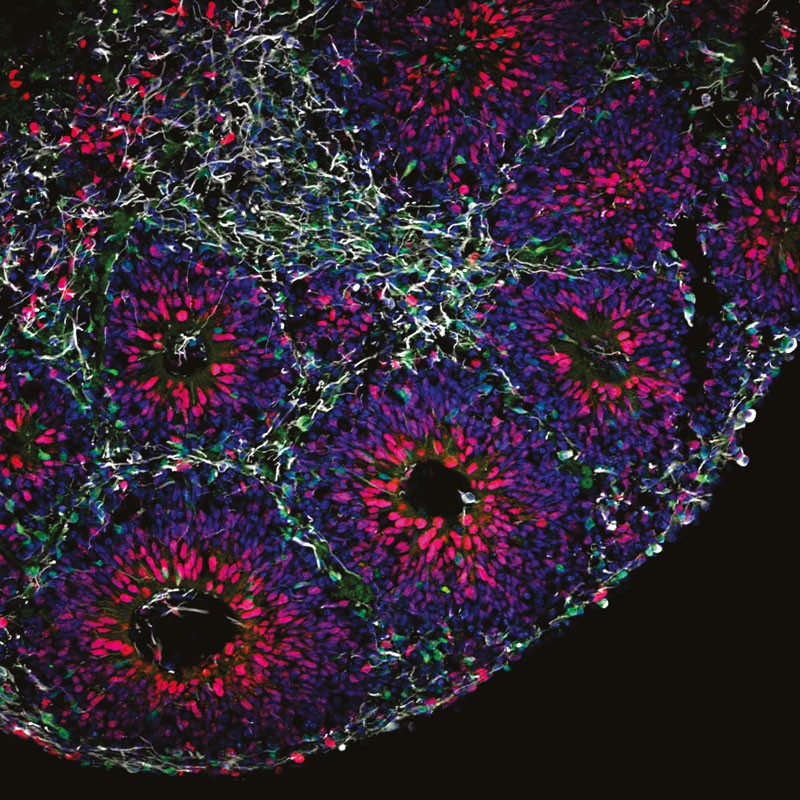
Micrograph of a developing brain organoid.
In developing human brain organoids, pre-neuronal cells (red) turn into neurons (green), which wire up into networks (white).
In any case, standard medical tests for consciousness are difficult to apply to brain cells grown in dishes, or disembodied animal brains. When Muotri suggested that his organoids’ firing patterns were just as complex as those seen in preterm infants, people were unsure what to make of that. Some researchers don’t consider the brain activity in a preterm infant to be complex enough to be classed as conscious. And organoids can’t blink or recoil from a painful stimulus, so they wouldn’t pass the clinical test for consciousness.
By contrast, it’s much more likely that an intact brain from a recently killed pig has the necessary structures for consciousness, as well as wiring created by memories and experiences the animal had while it was alive. “Thinking about a brain that has been filled with all this, it is hard to imagine that brain would be empty,” says Jeantine Lunshof, a philosopher and neuroethicist at Harvard University in Cambridge, Massachusetts. “What they can do in terms of thinking I don’t know, but it’s for sure not zero,” says Lunshof. Bringing a dead brain back to a semblance of life, as the Yale team did, might have the potential to restore a degree of consciousness, although the scientists took pains to avoid this by using chemical blocking agents that prevented brain-wide activity.
Researchers agree that they need to take the possibilities raised by these studies seriously. In October 2019, UCSD held a conference of about a dozen neuroscientists and philosophers, together with students and members of the public, with the intention of establishing and publishing an ethical framework for future experiments. But the paper has been delayed for months, partly because several of the authors could not agree on the basic requirements for consciousness.
Increasingly complex
Almost all scientists and ethicists agree that so far, nobody has created consciousness in the lab. But they are asking themselves what to watch out for, and which theories of consciousness might be most relevant. According to an idea called integrated information theory, for example, consciousness is a product of how densely neuronal networks are connected across the brain. The more neurons that interact with one another, the higher the degree of consciousness — a quantity known as phi. If phi is greater than zero, the organism is considered conscious.
Most animals reach this bar, according to the theory. Christof Koch, president of the Allen Institute for Brain Science in Seattle, Washington, doubts that any existing organoid could achieve this threshold, but concedes that a more advanced one might.
Other competing theories of consciousness require sensory input or coordinated electrical patterns across multiple brain regions. An idea known as global workspace theory, for instance, posits that the brain’s prefrontal cortex functions as a computer, processing sensory inputs and interpreting them to form a sense of being. Because organoids don’t have a prefrontal cortex and can’t receive input, they cannot become conscious. “Without input and output, the neurons may be talking with each other, but that doesn’t necessarily mean anything like human thought,” says Madeline Lancaster, a developmental biologist at the University of Cambridge, UK.
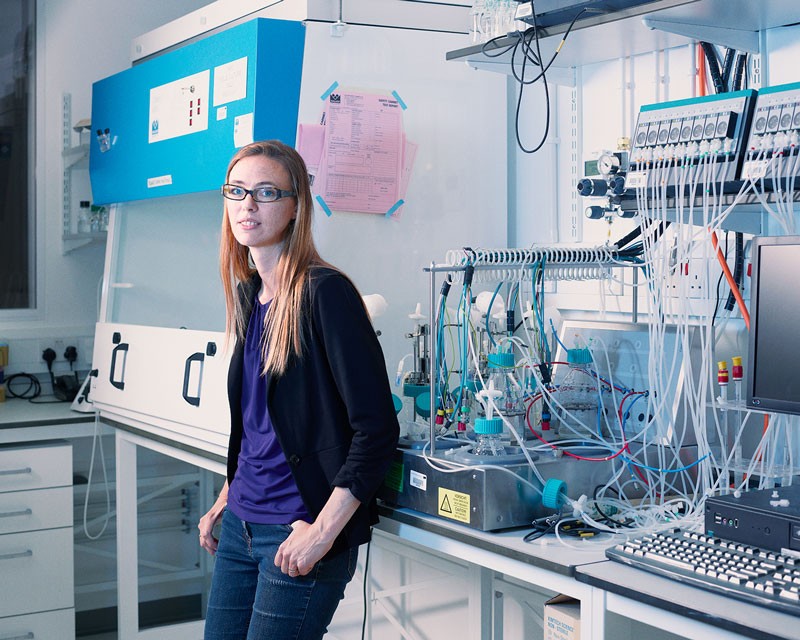
Connecting organoids to organs, however, could be a fairly simple task. In 2019, Lancaster’s team grew human brain organoids next to a mouse spinal column and back muscle. When nerves from the human organoid connected with the spinal column, the muscles began to spontaneously contract.
Developmental biologist Madeline Lancaster works with organoids to study brain organization and disorders in her lab at the University of Cambridge, UK.
Most organoids are built to reproduce only one portion of the brain — the cortex. But if they develop long enough and with the right kinds of growth factor, human stem cells spontaneously recreate many different parts of the brain, which then begin coordinating their electrical activity. In a study published in 2017, molecular biologist Paola Arlotta at Harvard University coaxed stem cells to develop into brain organoids composed of many different cell types, including light-sensitive cells like those found in the retina5. When exposed to light, neurons in the organoids began firing. But the fact that these cells were active doesn’t mean the organoids could see and process visual information, Arlotta says. It simply means that they could form the necessary circuits.
Arlotta and Lancaster think their organoids are too primitive to be conscious, because they lack the anatomical structures necessary to create complex EEG patterns. Still, Lancaster admits that for advanced organoids, it depends on the definition. “If you thought a fly was conscious, it’s conceivable that an organoid could be,” she says.
However, Lancaster and most other researchers think that something like a revitalized pig brain would be much more likely to achieve consciousness than an organoid. The team that did the work on the pig brains, led by neuroscientist Nenad Sestan, was trying to find new ways to revitalize organs, not to create consciousness. The researchers were able to get individual neurons or groups to fire and were careful to try and avoid the creation of widespread brain waves. Still, when Sestan’s team saw what looked like coordinated EEG activity in one of the brains, they immediately halted the project. Even after a neurology specialist confirmed that the pattern was not consistent with consciousness, the group anaesthetized the brains as a precautionary measure.
Sestan also contacted the US National Institutes of Health (NIH) for guidance on how to proceed. The agency’s neuroethics panel, including Lunshof and Insoo Hyun, a bioethicist at Case Western University in Cleveland, Ohio, assessed the work and agreed that Sestan should continue to anaesthetize the brains. But the panel hasn’t settled on more general regulations, and doesn’t routinely require a bioethics assessment for organoid proposals because its members think that consciousness is unlikely to arise. The NIH hasn’t arrived at a definition of consciousness, either. “It’s so flexible, everyone claims their own meaning,” Hyun says. “If it’s not clear we’re talking about the same thing, it’s a big problem for discourse.”
Neuroscientist Nenad Sestan used the BrainEx platform to restore neural activity in disembodied pig brains.
Fuzzy definitions
Some think it is futile to even try to identify consciousness in any sort of lab-maintained brain. “It’s just impossible to say meaningful things about what these bunches of brain cells could think or perceive, given we don’t understand consciousness,” says Steven Laureys, a neurologist at the University of Liège in Belgium, who pioneered some of the imaging-based measures of consciousness in people in a vegetative state. “We shouldn’t be too arrogant.” Further research should proceed very carefully, he says.
Laureys and others point out that the experience of an organoid is likely to be very different from that of a preterm infant, an adult human or a pig, and not directly comparable. Furthermore, the structures in an organoid might be too small to have their activity measured accurately, and similarities between the EEG patterns in organoids and preterm baby brains could be coincidental. Other scientists who work on brain organoids agree with Laureys that the question of whether a system is conscious could be unanswerable. Many avoid the idea entirely. “I don’t know why we would try to ask that question, because this system is not the human brain,” says Sergiu Pasça, a neuroscientist at Stanford University in California. “They’re made out of neurons, neurons have electrical activity, but we have to think carefully about how to compare them.”
Muotri wants his organoid systems to be comparable, in at least some ways, with human brains, so that he can study human disorders and find treatments. His motivation is personal: his 14-year-old son has epilepsy and autism. “He struggles hard in life,” Muotri says. Brain organoids are a promising avenue, because they recapitulate the earliest stages of brain wiring, which are impossible to study as a human embryo develops. But studying human brain disorders without a fully functioning brain, he says, is like studying a pancreas that doesn’t produce insulin. “To get there, I need a brain organoid model that really resembles a human brain. I might need an organoid that becomes conscious.”
Muotri says he is agnostic about which definition to use to decide whether an organoid reaches consciousness. At some point, he says, organoids might even be able to help researchers answer questions about how brains produce conscious states. For instance, mathematician Gabriel Silva at UCSD is studying neural activity in Muotri’s organoids to develop an algorithm that describes how the brain generates consciousness6. The goal of his project, which is partially funded by Microsoft, is to create an artificial system that works like human consciousness.
At the moment, there are no regulations in the United States or in Europe that would stop a researcher from creating consciousness. The National academies panel plans to release a report early next year, outlining the latest research and making a judgement on whether regulations are needed. Members plan to weigh in on questions such as whether to obtain people’s consent to develop their cells into brain organoids, and how to study and dispose of organoids humanely. The International Society for Stem Cell Research is also working on organoid guidelines, but is not addressing consciousness because it doesn’t think the science is there yet.
Hyun says that the NIH neuroethics panel has not yet seen any proposals to create complex, conscious organoids that would necessitate new guidelines. And Muotri says he doesn’t know of anyone else deliberately trying to create conscious organoids either, although a sufficiently complex organoid could, by some definitions, reach that status accidentally.
Still, Muotri and others say they would welcome some guidelines. These could include requiring scientists to justify the number of human brain organoids they use, to use them only for research that cannot be done in any other way, to restrict the amount of pain that can be inflicted on them, and to dispose of them humanely.
Having such advice in place ahead of time would help researchers weigh up the costs and benefits of creating conscious entities. And many researchers stress that such experiments have the potential to yield important insights. “There are truly conscious people out there with neurological disorders with no treatments,” Lancaster says. “If we did stop all of this research because of the philosophical thought experiment,” she adds, “that would be very detrimental to actual human beings who do need some new treatment.”
Treatments could still, however, be tested in brain organoids made using mouse stem cells , or in regular animal models. Such experiments could also inform discussions about the ethical use of human organoids. For instance, Hyun would like to see researchers compare the EEG patterns of mouse brain organoids with those of living mice, which might indicate how well human organoids recapitulate the human brain.
For his part, Muotri sees little difference between working on a human organoid or a lab mouse. “We work with animal models that are conscious and there are no problems,” he says. “We need to move forward and if it turns out they become conscious, to be honest I don’t see it as a big deal.”
Nature 586, 658-661 (2020)
doi:
https://doi.org/10.1038/d41586-020-02986-y
 www.nature.com
www.nature.com