IlostaMadge
Bluelighter
- Joined
- May 11, 2008
- Messages
- 514
Ripped straight from another forum, seems to be more at home here, nestling down amidst the psychonauts.
4-MEO-PCP looks to be of interest and appears to be legal. Any experience with it?
http://www.erowid.org/archive/rhodiu...4-meo-pcp.html
Would I be correct in predicting it's structure below: -
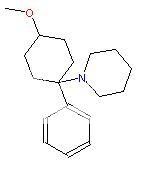
Would I be correct in assuming it's shorter duration would reduce the neurotoxicity?
Comments, critiscisms, thoughts or downright insults welcome...
4-MEO-PCP looks to be of interest and appears to be legal. Any experience with it?
http://www.erowid.org/archive/rhodiu...4-meo-pcp.html
4-methoxy PCP has been previously tested in animals and found to be somewhat less potent than PCP itself. Results with human volunteer confirm this. A rough estimate would be 70% as potent. This is still significant, considering how potent PCP is.
A possible advantage of this analog is decreased duration of effect. This is because the 4-methoxy group provides a site for metabolism and elimination of the drug. This is a significant point, because the extended action of PCP is a real disadvantage in cases of acute overdosage.
For those that are unfamiliar with the effects of these compounds, the experience is very hard to describe. The most commonly known compound that is directly comparable is ketamine. Ketamine's effects are quite similar to PCP and 4-methoxy PCP, but K. is much less potent.
Would I be correct in predicting it's structure below: -

Would I be correct in assuming it's shorter duration would reduce the neurotoxicity?
Comments, critiscisms, thoughts or downright insults welcome...


