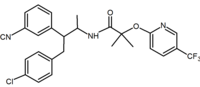Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
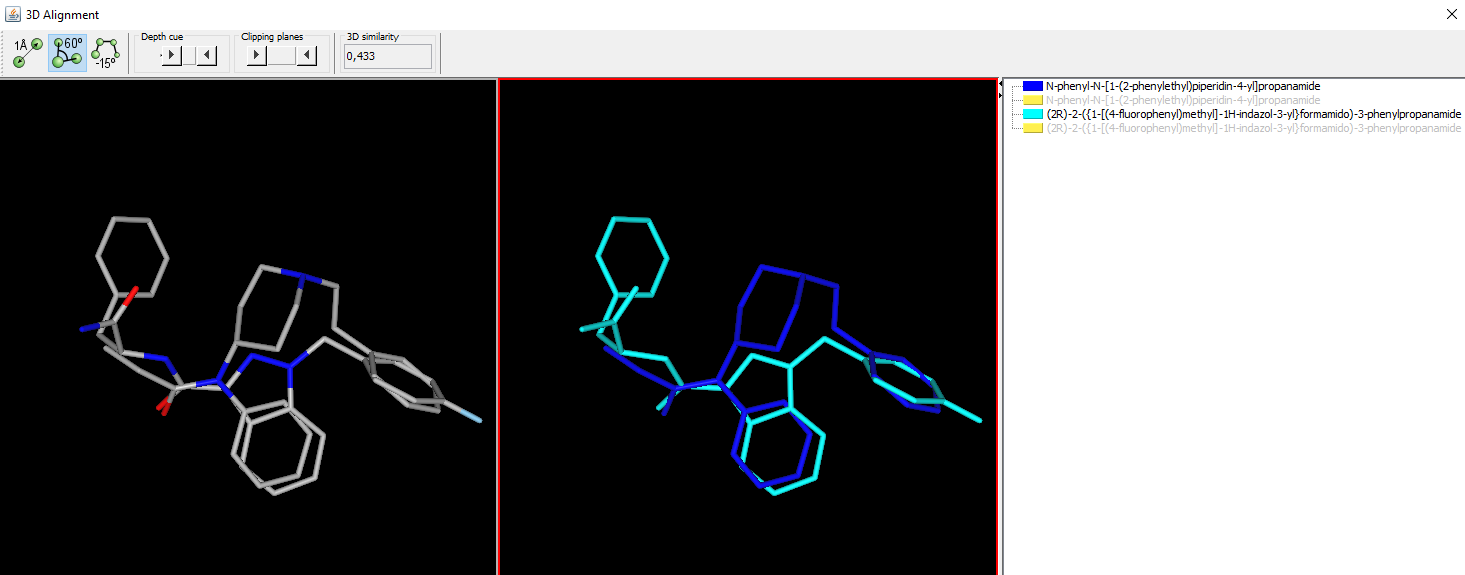
APP-FUBINACA
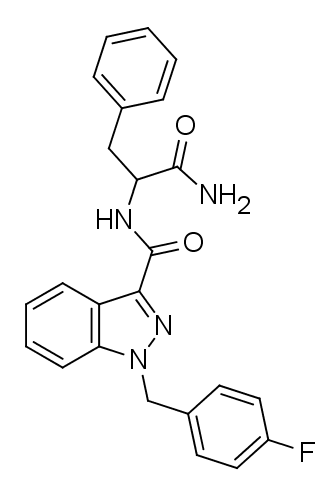
Seems fairly close in skeleton to the fentanyls:
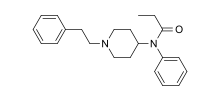
I know most known cannabinoid *antagonists* are also opioid inverse agonists. Since opioid agonists & antagonists are so close in structure, a cannabinoid antagonist that is an opioid agonist would be an interesting one.

Seems fairly close in skeleton to the fentanyls:

I know most known cannabinoid *antagonists* are also opioid inverse agonists. Since opioid agonists & antagonists are so close in structure, a cannabinoid antagonist that is an opioid agonist would be an interesting one.