Nervewing
Bluelighter
- Joined
- Jan 5, 2016
- Messages
- 243
Original post in its entirety here:
http://nervewing.blogspot.com/2021/01/new-drugs-dioxolanes.html
Per usual, accompanied with a flowchart.
Enlarged image of chart (right click>open image in new tab)
pdf download of chart
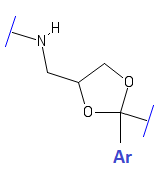
Generalized structure of a Dioxolane compound with NMDAr antagonist activity
[Introduction shared here, chem/pharm mumbo jumbo in spoiler]
Coming back to add a third addition to my series on the structure activity relationships of various compounds, I first touched on Arylcyclohexylamines and Diarylethylamines.
Dioxolanes dioxolanes how I would love to meet a dioxolane on the street some day! I have written a good bit on thesecompounds in the past. A good generalized but detailed introduction to this class of chemicals can be found in that article! These are curious characters that I personally believe hold a lot of potential. An untapped mineral vein, gleaming in the dark-
Overall, dioxolanes are fairly unpredictable when modified and there seem to be a range of cryptic patterns or lack thereof with their SAR, rife with exceptions and anomalies. It is for now, very hard (for me at least) to conjecture what may or may not be active.
Dioxolanes never had the distinction of making it to the recreational market. I have no knowledge of what the clandestine chemistry scene around dissociatives was like in the early 2010s but it seems like the only jump to a different class of molecules was driven by a UK ban on aryclyohexylamines. At that point there was a buffet of available options for the enterprising novel psychoactive chemical developer- The diarylethylamines ultimately took the prize, but the dioxolanes must have been considered then. Why did they remain relegated to the dustbin of history?
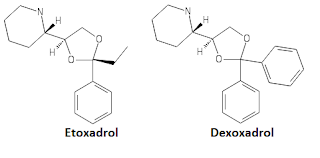
Only two were ever tested on humans and most literature on developing other Dioxolanes uses them as standards. These are Etoxadrol and Dexoxadrol.
When one delves into the medical literature on dioxolanes they encounter a lot of interesting comments on dioxolanes-These are excerpts pulled from my article on them, the citations can be found there!
“Open eyed hallucinations were not observed in post recovery, though subjects observed a "dream state" while anesthetized
"None of these dreams carried connotations of unhappiness to the individual; in fact, the majority were described as pleasant and/or unusual experiences. Consistent ideas of depersonalization, primarily of malinterpretations of self anatomical configurations, were a prominent symptom”
"The side effects caused due to the medication of Dexoxadrol are quite unusual and dangerous. It causes hallucinations and nightmares in the users. It has been reported that Dexoxadrol creates unpleasant conditions before the users. The dreams that came after the usage of this medicine range from pleasant to frightening. In dreams, it seems as they are in some other world that has no relation to the reality. But in most users, the results are outstanding rather than insane."
In most literature these effects can seem alarming, dysphoric, perhaps not particularly appealing. Perhaps it was this that turned research chemical designers off from developing them any further. Sensations described as vivid “dreams” or “nightmares” along with open eyed hallucinations were frequent. I hypothesize however, that what were referred to as dreams or nightmares were actually akin to dissociative “holes”- semiconscious states heavily laden with visuals, even visuals that bore a narrative- if this is the case then these would certainly be very interesting substances! It believe that at the time the medical literature simply lacked the vocabulary to do define those states as such. Other effects of the dioxolane experience were defined as “PCP-like”, while other side effects noted as concerning seemed to be exact descriptions of the qualities of a typical dissociative experience.
Nonetheless, this research was shortly after PCP had become an unexpected problem child in the world of anesthetic development. Seeing similar effects, it was possible that researchers quickly aborted trials and swept this class of chemicals under the rug to avoid repeating the trials and tribulations that came about through the lifespan of PCP. Meanwhile, the questionable, though I argue dated, descriptions of the experience from the contemporary literature may have turned future research chemical developers off from exploring this class of compounds further. Not only may they be very intriguing, but so far most have shown to be sufficiently potent, with dosages from 10-50 mg across the family depending on the compound and ROA. I personally believe that it is worthwhile to give this class of chemicals another shot!
What follows is all the dense chem and pharm mumbo jumbo:
So how do you go about designing novel chemicals in this class?
First, I must share the disclaimer I always share when dispensing this kid of information-
The safety profiles of the dioxolanes is entirely unknown. The only reference we have for doses is via IV, active doses by common oral, rectal, and intranasal means are entirely unknown. They have only seen limited human trials with only 2 compounds, which leaves a lot of unknowns. It is known that an accidental overdose of over 6x the intended dose was not fatal, though it yielded a long and difficult experience [7]. It appears that it is well tolerated for acute toxicity with minimal dangerous side effects. The effects of chronic use and chronic toxicity of dioxolanes is entirely unknown and this should be approached with the utmost caution. The risk of accidentally producing toxic byproducts from improper synthesis is also entirely unknown to me.
A warning about dissociatives in general, sources cited in this article:
“There are also a number of ways chronic toxicity is reported to present with NMDA antagonists, mainly neurotoxicity and cytotoxicity. In terms of neurotoxicity, there are the infamous Olney’s lesions, a form of brain damage, that has been observed in other animals, though they have still not officially been observed in humans yet [43]. However, a recent study reportedly observed some form of damage in the brain of extremely frequent users of ketamine [44]. The other main reported symptom that indicates toxicity is urinary toxicity [45, 46], supposedly a result of damage to the epithelial cells lining the bladder caused by direct toxicity from ketamine metabolites. This has so far only been officially reported with ketamine, though there are anecdotal reports of it occurring in frequent users of other dissociatives. There is also a potential for cognitive dysfunction from extreme repeated use of dissociatives, mostly in the form of “brain fog” and memory loss, though there is some literature on the matter [47].
These substances also carry the risk of generating dangerous behaviors that can be damaging to one’s life circumstances and relationships, both through the dangerous interplay of prohibition and substance, and in properties inherent to the chemicals themselves. One key risk is addiction- while physical dependence to dissociatives is significantly more rare than with other classes of substance, it is entirely possible and psychological dependence is commonly reported. Frequent usage significantly increases the chance of toxic effects or cognitive dysfunction presenting. Other substances, such as PCP, are notorious for causing intense mania that can push into psychosis, which can lead to violence, damaging relationships, and legal trouble. All of these risks are real and it is up to the user to determine what methods personally work best for mitigating them, including total abstinence if necessary.
I would suggest, in a perfect world (keep in mind this is all very handwaved, this actual process can be expensive, difficult, and extremely time consuming)- First, doing a virtual docking simulation of the compound. This of course is not a surefire way to determine activity, but can perhaps give warning of possible unexpected activity or help to rule out certain options as being less viable. The compound can be synthesized from there, at which point it must be properly characterized via NMR and GC/MS analysis. From there, an in-vitro receptor affinity study can be done to confirm or deny certain targeted activities in nerve cells in comparison to familiar reference compounds, like PCP, Ketamine, MK-801 or Morphine. The safest step from there would be in-vivo studies in animals, also compared to a control group of reference compounds. Behavioral tests can be done for comparison to any references, and drug substitution tests can help indicate similarity to the references. There is a huge variety of animal tests that can be done in combination with each other and with various controls to really narrow down possible mechanism of action depending on what a researcher has at their disposal. In-vivo tests also help to determine an mg/kg dosage range and possible acute or chronic toxicity, or even an LD-50. Only after it has been presumed nontoxic and its likely activities have been characterized should one even consider human testing. This must also be done in the context of extremely precise doses, titrated upwards from a microgram range, with the subject physically monitored by a healthcare professional. If you want to get really fancy, this can be performed in a double blind test with a placebo.
Of course not all those processes or resources are available to every researcher. Those are all long, difficult, expensive processes that may require specialty equipment, facilities, and faculty. Many researchers of psychoactive compounds have opted to skip some or most or all of those steps, and prohibition absolutely makes obtaining any of those resources extremely difficult. I would recommend approaching with maximum caution, but I’m also not the boss of anyone and can’t make anyone do anything, and understand how the spirit of curiosity can sometimes overcome a lack of available resources. Ideally a team of researchers could easily have infrastructure to efficiently run multiple compounds through that gauntlet of safety determinations. But this world is less than ideal. Please just for the love of god, be safe, be smart, be responsible.”
Here’s a handy flowchart to assist you!
For an enlarged version of this image: (right click>open image in new tab)
For a download of a pdf of this chart: https://gofile.io/d/h05j2m

What are the exact details of the SAR of this class of compounds and why did I include what I did? Let’s get into it.
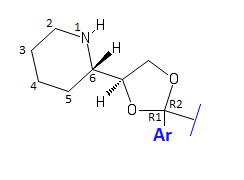
Working our way down from the top of the molecule-
Starting with the amine: In the dioxolanes that have seen in vivo usage, this is a 2-piperidine. One attempt was made however, to replace this with a simple primary amine on an ethylamine chain extending from the dioxolane. In this case, a trans analogue of etoxadrol with an ethylamine was one of the only compounds to show any activity, and it was much less than that of base etoxadrol [1]. Even more promising was the 2S, 4R enantiomer of this same compound, which saw increased potency, though not quite as much as etoxadrol [1]. This has broader implications for potentially attempting other amines beyond the standard 2-piperidine. It appears there needs to be at least 2 carbons between the dioxolane ring and the nitrogen to conserve activity- necessitating an ethylamine. While the primary ethylamine has been demonstrated to be active in vitro, a secondary ethylamine (as seen in the standard piperidine ring) may also be active, yielding an immense variety of potentially active variations. Beyond that, a tertiary amine appears inactive [6]. A Pyrollidine instead of a piperidine has also been attempted but was found to be inactive [6]. So it all seems quite fickle and hard to predict.
Working with a piperidine however, many other little tidbits are known. For one, the nitrogen must remain as a secondary amine- as mentioned above, adding substitutions to turn it into a tertiary amine greatly decreases activity [6]. Secondly, NMDAr antagonist effects are conserved (and potency either retained or increased!) with very specific substitutions on the 4 position of the piperidine ring. It seems to be fickle for which substitutions it’ll accept however. So far it has been demonstrated that a Dexoxadrol substituted with a 4-Fluorine on the piperidine is even more potent in vitro than Dexoxadrol alone [3]. A Difluoro substitution also appeared sufficiently active [3]. A Hydroxy group is also tolerated [2]. The 4-fluoro and 4-Hydroxy analogues of Dexoxadrol have been named WMS-2539 and WMS-2508 respectively. Meanwhile, a double bonded oxygen, forming a 4-piperidone, is hardly active, as is a 4-methoxy group, as is any kind of 4-amino group [5] [2]. This makes it hard to guess what can and cannot be placed there (for me at least). Perhaps other halogens would work, either in mono- or di- form. Perhaps a methyl group though it seems unlikely that anything extending beyond that would remain active, ass activity on those substitutions is hypothesized to be correlated with bulk be (by my conjecture) that a substitution larger than ~30 u will not be tolerated (though multiple substitutions can still be affixed to the same spot, even if they cumulatively have a mass greater than 30 u!). The amino substitution has less bulk than that- but perhaps there is something in the unique properties of the nitrogen that precludes activity. As for substitutions on other parts of the piperidine ring? They haven’t been attempted and so far no one knows how they might behave.
Next we move on to the next component- the eponymous Dioxolane ring. Several studies attempted to expand the dioxolane ring to a 6-member Dioxane ring. It was apparent that this greatly decreased activity in most circumstances [1] [6] [5]. The Dioxolane ring is important.
There is of course always an exception- if the piperidine ring is replaced with an ethylamine with a primary amine at the end, then NMDAr antagonist activity is yet again retained. This suggests an entirely new class of compound closely related to the dioxolanes called the dioxanes- a 6 member 2-oxygen ring (as a 1,3-dioxane) with an ethylamine chain instead of a piperidine [5].
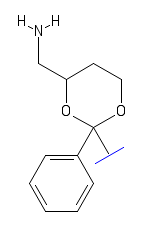
Structure of a hypothetical 1,3-dioxane based compound with a primary amine
So now we move onto the next options at our disposal- an aromatic ring and some other functional group bonded to the same quaternary carbon on the dioxolane ring. Etoxadrol sees that functional group as an ethyl, while dexoxadrol sees a second phenyl ring. In all drugs that have been attempted in vivo, the aromatic rings were phenyl rings. However, one example that showed NMDAr antagonist activity in vitro and equivalent or slightly less potency to etoxadrol was an analogue of etoxadrol with the phenyl ring replaced with a thiophene, just as the thiophene would differentiate TCP from PCP. The 2-Thiophene showed less potency than the 3-Thiophene [6]. So in any variation of a dioxolane, it is feasible to replace the lower phenyl ring with at the very least a thiophene, if not other aromatic rings. As activity can be conserved with two aromatic rings bonded to the same spot, different aromatic rings could even be mixed and matched if it was synthetically feasible. The bottom line however is that there must be at least one aromatic ring. As for the other spot on that carbon?
The golden standard has been a simple ethyl group. One study demonstrated in vitro that other alkane chains, from a propyl to a butyl, also conserved an appreciable amount of activity [5]. The most potent compounds had the phenyl ring matched with either a propyl or isopropyl group [5]. It is unknown what else may be possible: Halogenated groups, ethers and thioalkanes, esters, other aromatic rings, various heterocycles- there’s nothing that indicates to me that these wouldn’t be possible!
The last option for modification is adding substitutions to the aromatic ring (in this case, and rather by default, a phenyl ring). 2-Cl, 3-Cl, 2-F, 3-F, 3-OH, 2-OH substitutions retained an appreciable level of activity [5] [6]. Activity doesn’t seem consistent across position no matter the class of substitution-the hydroxy substitution (already fairly impotent), sees an even further drop in activity on the 3-position, as does a Fluorine substitution (though they still show an appreciable level of activity, the drop in potency is still remarkable!) [5] [6]. Substitutions on the 4 position were consistently inactive [5]. This suggests that the main suite of substitutions seen on dissociatives will conserve activity if on the 2 and/or 3 position variably, one way or another. Perhaps the usual alkanes, and alkoxy groups could also retain activity there. The most off-the-wall substitution that saw retained activity was working a diphenylazepine (that is 2 phenyl groups on an aromatic 7 member ring) onto the R1 and R2 positions [6].
As far as stereochemistry is concerned, it appears that only the (+)-enantiomer of any enantiomeric compound is active as an NMDA antagonist. Racemic mixtures will see the potency cut in half and the (-)-enantiomer has so far been demonstrated as inactive [4].
As for nomenclature? No logical patterns have been established- the 2 named compounds (Etoxadrol and Dexoxadrol). have an informal naming structure, with one of them, Dexoxadrol indicating that it is merely the dextro-isomer of the parent compound, Dioxoadrol. The names give little indication of structure, beyond the mention of an ethyl group in Etoxadrol. Thus for compounds developed further, a new nomenclatural scheme will have to be developed.
The name can be prefixed with the relevant substitutions that have been added to the molecule. The suffix -oxadrol, can be kept, and if a piperidine as the amine is assumed to be the “default”, then modifications of the amine can be worked into this name. So if there’s an N-propyl tertiary amine, for example, the compound would bare the suffix of “N-propyloxadrol”. The two moieties on R1 and R2 can then be worked into the name. The name of the R1 aromatic ring should always come second. Thus, Etoxadrol would in this schematic be renamed to Etphenyloxadrol. Dexoxadrol would be Diphenyloxadrol. If for example, a methoxy group or a chlorine was paired with say, a thiophene, the names would respectively be Methoxythiophenyloxadrol or Chorothiophenyloxadrol. If you were to pair these with, for the sake of example, a sec-butyl secondary amine, they would yield the unwieldly names Methoxythiophenyl-N-sec-butyloxadrol or Chlorothiophenyl-N-sec-butyloxadrol respectively. If an oxane ring is used instead of a dioxalane, the suffix can be modified from -oxadrol to -oxanadrol.
Sources and further reading:
[1]- Aepkers M, Wünsch B (2005) Structure-affinity relationship studies of non-competitive NMDA receptor antagonists derived from dexoxadrol and etoxadrol. Bioorg Med Chem. 13(24):6836-49.
[2]-Banerjee A, Fröhlich R, Schepmanna D, Wünsch B (2010) Synthesis and NMDA receptor affinity of dexoxadrol analogues with modifications in position 4 of the piperidine ring. Med. Chem. Commun. 1:87-102
[3]-Banerjee A, Schepmann D, Köhler J, Würthwein E-U, Wünsch B, (2010) Synthesis and SAR studies of chiral non-racemic dexoxadrol analogues as uncompetitive NMDA receptor antagonists. Bioorganic & Medicinal Chemistry 18(22):7855-7867
[4]- Jacobson AE, Harrison EA, Mattson MV, Rafferty MF, Rice KC, Woods JH, Winger G, Solomon RE, Lessor RA, Silverton JV (1987) Enantiomeric and diastereomeric dioxadrols: behavioral, biochemical and chemical determination of the configuration necessary for phencyclidine-like properties. Journal of Pharmacology and Experimental Therapeutics 243(1):110-117
[5]- Sax M, Fröhlich R. Schepmann D, Wünsch B (2008) Synthesis and NMDA Receptor Affinity of Ring and Side Chain Homologues of Dexoxadrol. European Journal of Organic Chemistry: 6015-6028
[6]- Thurkauf A, Mattson MV, Richardson S, Mirsadeghi S, Ornstein PL, Harrison EA Jr, Rice KC, Jacobson AE, Monn JA (1992) Analogues of the dioxolanes dexoxadrol and etoxadrol as potential phencyclidine-like agents. Synthesis and structure-activity relationships. J Med Chem. 35(8):1323-9
[7] -Wilson RD, Traber DL, Barratt E, Creson DL, Schmitt RC, Allen CR (1970) Evaluation of CL-1848C: a new dissociative anesthetic in normal human volunteers. Anesth Analg. 49(2):236‐241.
http://nervewing.blogspot.com/2021/01/new-drugs-dioxolanes.html
Per usual, accompanied with a flowchart.
Enlarged image of chart (right click>open image in new tab)
pdf download of chart

Generalized structure of a Dioxolane compound with NMDAr antagonist activity
[Introduction shared here, chem/pharm mumbo jumbo in spoiler]
Coming back to add a third addition to my series on the structure activity relationships of various compounds, I first touched on Arylcyclohexylamines and Diarylethylamines.
Dioxolanes dioxolanes how I would love to meet a dioxolane on the street some day! I have written a good bit on thesecompounds in the past. A good generalized but detailed introduction to this class of chemicals can be found in that article! These are curious characters that I personally believe hold a lot of potential. An untapped mineral vein, gleaming in the dark-
Overall, dioxolanes are fairly unpredictable when modified and there seem to be a range of cryptic patterns or lack thereof with their SAR, rife with exceptions and anomalies. It is for now, very hard (for me at least) to conjecture what may or may not be active.
Dioxolanes never had the distinction of making it to the recreational market. I have no knowledge of what the clandestine chemistry scene around dissociatives was like in the early 2010s but it seems like the only jump to a different class of molecules was driven by a UK ban on aryclyohexylamines. At that point there was a buffet of available options for the enterprising novel psychoactive chemical developer- The diarylethylamines ultimately took the prize, but the dioxolanes must have been considered then. Why did they remain relegated to the dustbin of history?
Only two were ever tested on humans and most literature on developing other Dioxolanes uses them as standards. These are Etoxadrol and Dexoxadrol.

When one delves into the medical literature on dioxolanes they encounter a lot of interesting comments on dioxolanes-These are excerpts pulled from my article on them, the citations can be found there!
“Open eyed hallucinations were not observed in post recovery, though subjects observed a "dream state" while anesthetized
"None of these dreams carried connotations of unhappiness to the individual; in fact, the majority were described as pleasant and/or unusual experiences. Consistent ideas of depersonalization, primarily of malinterpretations of self anatomical configurations, were a prominent symptom”
"The side effects caused due to the medication of Dexoxadrol are quite unusual and dangerous. It causes hallucinations and nightmares in the users. It has been reported that Dexoxadrol creates unpleasant conditions before the users. The dreams that came after the usage of this medicine range from pleasant to frightening. In dreams, it seems as they are in some other world that has no relation to the reality. But in most users, the results are outstanding rather than insane."
In most literature these effects can seem alarming, dysphoric, perhaps not particularly appealing. Perhaps it was this that turned research chemical designers off from developing them any further. Sensations described as vivid “dreams” or “nightmares” along with open eyed hallucinations were frequent. I hypothesize however, that what were referred to as dreams or nightmares were actually akin to dissociative “holes”- semiconscious states heavily laden with visuals, even visuals that bore a narrative- if this is the case then these would certainly be very interesting substances! It believe that at the time the medical literature simply lacked the vocabulary to do define those states as such. Other effects of the dioxolane experience were defined as “PCP-like”, while other side effects noted as concerning seemed to be exact descriptions of the qualities of a typical dissociative experience.
Nonetheless, this research was shortly after PCP had become an unexpected problem child in the world of anesthetic development. Seeing similar effects, it was possible that researchers quickly aborted trials and swept this class of chemicals under the rug to avoid repeating the trials and tribulations that came about through the lifespan of PCP. Meanwhile, the questionable, though I argue dated, descriptions of the experience from the contemporary literature may have turned future research chemical developers off from exploring this class of compounds further. Not only may they be very intriguing, but so far most have shown to be sufficiently potent, with dosages from 10-50 mg across the family depending on the compound and ROA. I personally believe that it is worthwhile to give this class of chemicals another shot!
What follows is all the dense chem and pharm mumbo jumbo:

So how do you go about designing novel chemicals in this class?
First, I must share the disclaimer I always share when dispensing this kid of information-
The safety profiles of the dioxolanes is entirely unknown. The only reference we have for doses is via IV, active doses by common oral, rectal, and intranasal means are entirely unknown. They have only seen limited human trials with only 2 compounds, which leaves a lot of unknowns. It is known that an accidental overdose of over 6x the intended dose was not fatal, though it yielded a long and difficult experience [7]. It appears that it is well tolerated for acute toxicity with minimal dangerous side effects. The effects of chronic use and chronic toxicity of dioxolanes is entirely unknown and this should be approached with the utmost caution. The risk of accidentally producing toxic byproducts from improper synthesis is also entirely unknown to me.
A warning about dissociatives in general, sources cited in this article:
“There are also a number of ways chronic toxicity is reported to present with NMDA antagonists, mainly neurotoxicity and cytotoxicity. In terms of neurotoxicity, there are the infamous Olney’s lesions, a form of brain damage, that has been observed in other animals, though they have still not officially been observed in humans yet [43]. However, a recent study reportedly observed some form of damage in the brain of extremely frequent users of ketamine [44]. The other main reported symptom that indicates toxicity is urinary toxicity [45, 46], supposedly a result of damage to the epithelial cells lining the bladder caused by direct toxicity from ketamine metabolites. This has so far only been officially reported with ketamine, though there are anecdotal reports of it occurring in frequent users of other dissociatives. There is also a potential for cognitive dysfunction from extreme repeated use of dissociatives, mostly in the form of “brain fog” and memory loss, though there is some literature on the matter [47].
These substances also carry the risk of generating dangerous behaviors that can be damaging to one’s life circumstances and relationships, both through the dangerous interplay of prohibition and substance, and in properties inherent to the chemicals themselves. One key risk is addiction- while physical dependence to dissociatives is significantly more rare than with other classes of substance, it is entirely possible and psychological dependence is commonly reported. Frequent usage significantly increases the chance of toxic effects or cognitive dysfunction presenting. Other substances, such as PCP, are notorious for causing intense mania that can push into psychosis, which can lead to violence, damaging relationships, and legal trouble. All of these risks are real and it is up to the user to determine what methods personally work best for mitigating them, including total abstinence if necessary.
I would suggest, in a perfect world (keep in mind this is all very handwaved, this actual process can be expensive, difficult, and extremely time consuming)- First, doing a virtual docking simulation of the compound. This of course is not a surefire way to determine activity, but can perhaps give warning of possible unexpected activity or help to rule out certain options as being less viable. The compound can be synthesized from there, at which point it must be properly characterized via NMR and GC/MS analysis. From there, an in-vitro receptor affinity study can be done to confirm or deny certain targeted activities in nerve cells in comparison to familiar reference compounds, like PCP, Ketamine, MK-801 or Morphine. The safest step from there would be in-vivo studies in animals, also compared to a control group of reference compounds. Behavioral tests can be done for comparison to any references, and drug substitution tests can help indicate similarity to the references. There is a huge variety of animal tests that can be done in combination with each other and with various controls to really narrow down possible mechanism of action depending on what a researcher has at their disposal. In-vivo tests also help to determine an mg/kg dosage range and possible acute or chronic toxicity, or even an LD-50. Only after it has been presumed nontoxic and its likely activities have been characterized should one even consider human testing. This must also be done in the context of extremely precise doses, titrated upwards from a microgram range, with the subject physically monitored by a healthcare professional. If you want to get really fancy, this can be performed in a double blind test with a placebo.
Of course not all those processes or resources are available to every researcher. Those are all long, difficult, expensive processes that may require specialty equipment, facilities, and faculty. Many researchers of psychoactive compounds have opted to skip some or most or all of those steps, and prohibition absolutely makes obtaining any of those resources extremely difficult. I would recommend approaching with maximum caution, but I’m also not the boss of anyone and can’t make anyone do anything, and understand how the spirit of curiosity can sometimes overcome a lack of available resources. Ideally a team of researchers could easily have infrastructure to efficiently run multiple compounds through that gauntlet of safety determinations. But this world is less than ideal. Please just for the love of god, be safe, be smart, be responsible.”
Here’s a handy flowchart to assist you!
For an enlarged version of this image: (right click>open image in new tab)
For a download of a pdf of this chart: https://gofile.io/d/h05j2m

What are the exact details of the SAR of this class of compounds and why did I include what I did? Let’s get into it.
Working our way down from the top of the molecule-
Starting with the amine: In the dioxolanes that have seen in vivo usage, this is a 2-piperidine. One attempt was made however, to replace this with a simple primary amine on an ethylamine chain extending from the dioxolane. In this case, a trans analogue of etoxadrol with an ethylamine was one of the only compounds to show any activity, and it was much less than that of base etoxadrol [1]. Even more promising was the 2S, 4R enantiomer of this same compound, which saw increased potency, though not quite as much as etoxadrol [1]. This has broader implications for potentially attempting other amines beyond the standard 2-piperidine. It appears there needs to be at least 2 carbons between the dioxolane ring and the nitrogen to conserve activity- necessitating an ethylamine. While the primary ethylamine has been demonstrated to be active in vitro, a secondary ethylamine (as seen in the standard piperidine ring) may also be active, yielding an immense variety of potentially active variations. Beyond that, a tertiary amine appears inactive [6]. A Pyrollidine instead of a piperidine has also been attempted but was found to be inactive [6]. So it all seems quite fickle and hard to predict.
Working with a piperidine however, many other little tidbits are known. For one, the nitrogen must remain as a secondary amine- as mentioned above, adding substitutions to turn it into a tertiary amine greatly decreases activity [6]. Secondly, NMDAr antagonist effects are conserved (and potency either retained or increased!) with very specific substitutions on the 4 position of the piperidine ring. It seems to be fickle for which substitutions it’ll accept however. So far it has been demonstrated that a Dexoxadrol substituted with a 4-Fluorine on the piperidine is even more potent in vitro than Dexoxadrol alone [3]. A Difluoro substitution also appeared sufficiently active [3]. A Hydroxy group is also tolerated [2]. The 4-fluoro and 4-Hydroxy analogues of Dexoxadrol have been named WMS-2539 and WMS-2508 respectively. Meanwhile, a double bonded oxygen, forming a 4-piperidone, is hardly active, as is a 4-methoxy group, as is any kind of 4-amino group [5] [2]. This makes it hard to guess what can and cannot be placed there (for me at least). Perhaps other halogens would work, either in mono- or di- form. Perhaps a methyl group though it seems unlikely that anything extending beyond that would remain active, ass activity on those substitutions is hypothesized to be correlated with bulk be (by my conjecture) that a substitution larger than ~30 u will not be tolerated (though multiple substitutions can still be affixed to the same spot, even if they cumulatively have a mass greater than 30 u!). The amino substitution has less bulk than that- but perhaps there is something in the unique properties of the nitrogen that precludes activity. As for substitutions on other parts of the piperidine ring? They haven’t been attempted and so far no one knows how they might behave.
Next we move on to the next component- the eponymous Dioxolane ring. Several studies attempted to expand the dioxolane ring to a 6-member Dioxane ring. It was apparent that this greatly decreased activity in most circumstances [1] [6] [5]. The Dioxolane ring is important.
There is of course always an exception- if the piperidine ring is replaced with an ethylamine with a primary amine at the end, then NMDAr antagonist activity is yet again retained. This suggests an entirely new class of compound closely related to the dioxolanes called the dioxanes- a 6 member 2-oxygen ring (as a 1,3-dioxane) with an ethylamine chain instead of a piperidine [5].

Structure of a hypothetical 1,3-dioxane based compound with a primary amine
So now we move onto the next options at our disposal- an aromatic ring and some other functional group bonded to the same quaternary carbon on the dioxolane ring. Etoxadrol sees that functional group as an ethyl, while dexoxadrol sees a second phenyl ring. In all drugs that have been attempted in vivo, the aromatic rings were phenyl rings. However, one example that showed NMDAr antagonist activity in vitro and equivalent or slightly less potency to etoxadrol was an analogue of etoxadrol with the phenyl ring replaced with a thiophene, just as the thiophene would differentiate TCP from PCP. The 2-Thiophene showed less potency than the 3-Thiophene [6]. So in any variation of a dioxolane, it is feasible to replace the lower phenyl ring with at the very least a thiophene, if not other aromatic rings. As activity can be conserved with two aromatic rings bonded to the same spot, different aromatic rings could even be mixed and matched if it was synthetically feasible. The bottom line however is that there must be at least one aromatic ring. As for the other spot on that carbon?
The golden standard has been a simple ethyl group. One study demonstrated in vitro that other alkane chains, from a propyl to a butyl, also conserved an appreciable amount of activity [5]. The most potent compounds had the phenyl ring matched with either a propyl or isopropyl group [5]. It is unknown what else may be possible: Halogenated groups, ethers and thioalkanes, esters, other aromatic rings, various heterocycles- there’s nothing that indicates to me that these wouldn’t be possible!
The last option for modification is adding substitutions to the aromatic ring (in this case, and rather by default, a phenyl ring). 2-Cl, 3-Cl, 2-F, 3-F, 3-OH, 2-OH substitutions retained an appreciable level of activity [5] [6]. Activity doesn’t seem consistent across position no matter the class of substitution-the hydroxy substitution (already fairly impotent), sees an even further drop in activity on the 3-position, as does a Fluorine substitution (though they still show an appreciable level of activity, the drop in potency is still remarkable!) [5] [6]. Substitutions on the 4 position were consistently inactive [5]. This suggests that the main suite of substitutions seen on dissociatives will conserve activity if on the 2 and/or 3 position variably, one way or another. Perhaps the usual alkanes, and alkoxy groups could also retain activity there. The most off-the-wall substitution that saw retained activity was working a diphenylazepine (that is 2 phenyl groups on an aromatic 7 member ring) onto the R1 and R2 positions [6].
As far as stereochemistry is concerned, it appears that only the (+)-enantiomer of any enantiomeric compound is active as an NMDA antagonist. Racemic mixtures will see the potency cut in half and the (-)-enantiomer has so far been demonstrated as inactive [4].
As for nomenclature? No logical patterns have been established- the 2 named compounds (Etoxadrol and Dexoxadrol). have an informal naming structure, with one of them, Dexoxadrol indicating that it is merely the dextro-isomer of the parent compound, Dioxoadrol. The names give little indication of structure, beyond the mention of an ethyl group in Etoxadrol. Thus for compounds developed further, a new nomenclatural scheme will have to be developed.
The name can be prefixed with the relevant substitutions that have been added to the molecule. The suffix -oxadrol, can be kept, and if a piperidine as the amine is assumed to be the “default”, then modifications of the amine can be worked into this name. So if there’s an N-propyl tertiary amine, for example, the compound would bare the suffix of “N-propyloxadrol”. The two moieties on R1 and R2 can then be worked into the name. The name of the R1 aromatic ring should always come second. Thus, Etoxadrol would in this schematic be renamed to Etphenyloxadrol. Dexoxadrol would be Diphenyloxadrol. If for example, a methoxy group or a chlorine was paired with say, a thiophene, the names would respectively be Methoxythiophenyloxadrol or Chorothiophenyloxadrol. If you were to pair these with, for the sake of example, a sec-butyl secondary amine, they would yield the unwieldly names Methoxythiophenyl-N-sec-butyloxadrol or Chlorothiophenyl-N-sec-butyloxadrol respectively. If an oxane ring is used instead of a dioxalane, the suffix can be modified from -oxadrol to -oxanadrol.
Sources and further reading:
[1]- Aepkers M, Wünsch B (2005) Structure-affinity relationship studies of non-competitive NMDA receptor antagonists derived from dexoxadrol and etoxadrol. Bioorg Med Chem. 13(24):6836-49.
[2]-Banerjee A, Fröhlich R, Schepmanna D, Wünsch B (2010) Synthesis and NMDA receptor affinity of dexoxadrol analogues with modifications in position 4 of the piperidine ring. Med. Chem. Commun. 1:87-102
[3]-Banerjee A, Schepmann D, Köhler J, Würthwein E-U, Wünsch B, (2010) Synthesis and SAR studies of chiral non-racemic dexoxadrol analogues as uncompetitive NMDA receptor antagonists. Bioorganic & Medicinal Chemistry 18(22):7855-7867
[4]- Jacobson AE, Harrison EA, Mattson MV, Rafferty MF, Rice KC, Woods JH, Winger G, Solomon RE, Lessor RA, Silverton JV (1987) Enantiomeric and diastereomeric dioxadrols: behavioral, biochemical and chemical determination of the configuration necessary for phencyclidine-like properties. Journal of Pharmacology and Experimental Therapeutics 243(1):110-117
[5]- Sax M, Fröhlich R. Schepmann D, Wünsch B (2008) Synthesis and NMDA Receptor Affinity of Ring and Side Chain Homologues of Dexoxadrol. European Journal of Organic Chemistry: 6015-6028
[6]- Thurkauf A, Mattson MV, Richardson S, Mirsadeghi S, Ornstein PL, Harrison EA Jr, Rice KC, Jacobson AE, Monn JA (1992) Analogues of the dioxolanes dexoxadrol and etoxadrol as potential phencyclidine-like agents. Synthesis and structure-activity relationships. J Med Chem. 35(8):1323-9
[7] -Wilson RD, Traber DL, Barratt E, Creson DL, Schmitt RC, Allen CR (1970) Evaluation of CL-1848C: a new dissociative anesthetic in normal human volunteers. Anesth Analg. 49(2):236‐241.



