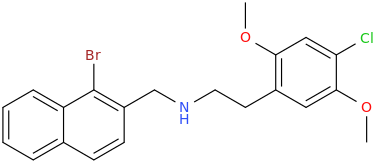
2C-C-N2Nap1Br or 25C-N2Nap1Br
*********************************************
2C-C-N2Nap1Br or 25C-N2Nap1Br* date 151025
*********************************************
*nomenclature follows the rules of Michael R. Braden et al: Molecular interactions of serotonin 5-HT2A receptor residues Phe339 and Phe340 with super-potent N-benzyl phenethylamine agonists. Department of Medicinal Chemistry and Molecular Pharmacology School of Pharmacy and Pharmaceutical Sciences, Purdue University, West Lafayette, IN 47907;
IUPAC-Name:
N-[(1-bromo-2-naphthyl)methyl]-2-(4-chloro-2,5-dimethoxy-phenyl)ethanamine
Structure:
Trial 1:
Salt: Sulfate
Dose of free base [nMol/kg]; (M=434.7 g/Mol): 1.459 nMol/kg
Dose [µg] of free base related to a 75 kg body: 48 µg
Route of Administration: s.l. (sub lingual)
Duration: ~ 4 h
Notes: 8.June 2015; 14:00h ~60µg sulfate in ~15µL etOH s.l.; ~16:00h smooth weak effect, beeing dull a littl bit, something not on a comfy couch. 17:30h seems to be finished, a touch of beeing narcotic. 23:00h had a nice evening. 23:30h to bed, incredible pictures during falling asleep. Above threshold.
Trial 3:
Salt: Sulfate
Dose of free base [nMol/kg]; (M=434.7 g/Mol): 4.377 nMol/kg
Dose [µg] of free base related to a 75 kg body: 143 µg
Route of Administration: s.l.
Duration: ~ 6 h
Notes: 9.July 2015; 17:30h ~180µg s.l., then nordic walking 2h; very nice walking, clear thoughts. 21:00h turned on, but hardly (+); nice relaxed evening. 23:00h to bed, good sleep, but was aware of going on at a low level all the night, so sleep was a little bit not as deep as usual.
Trial 4:
Salt: Sulfate
Dose of free base [nMol/kg]; (M=434.7 g/Mol): 9.727 nMol/kg
Dose [µg] of free base related to a 75 kg body: 317 µg
Route of Administration: s.l.
Duration: ~ 10(?) h
Notes: 5.Sept.2015; 8:30h ~400µg sulfate s.l.; 9:00h it works! 9:30h nordic walking quite nice, but deeper emotions (normal experience of nature) failed, despite more clear sight; all a little bit subdued mood. 12:00h seems coming down; 13:00h comes again in waves; guitar-music, very impressive, analytical insights: concerning the very early years of my childhood. 15:30h into the garden, very impressive nature, clouds, sun, wind, flowers. On the whole: subdued mood (but that may be owed to the Trimipramin (an Antidepressive) that I take (low dose 7mg/d) against my depressions since some weeks.). (+) and sometimes (++) (on couch with music). Nice afterglow. 24:00h: still not be sleepy. Later on, sleep was a liitle bit not as deep as usual.
More details can be seen here (Russian Hyperlab, they accept English too):
https://www.hyperlab.info/inv/index.php?act=ST&f=17&t=31263
(and click "continue" further down)
Own comments need registration i.e. under
https://www.hyperlab.info/inv/index.php?act=ST&f=17&t=30352&lang=en
The links are SURE! despite warnings.