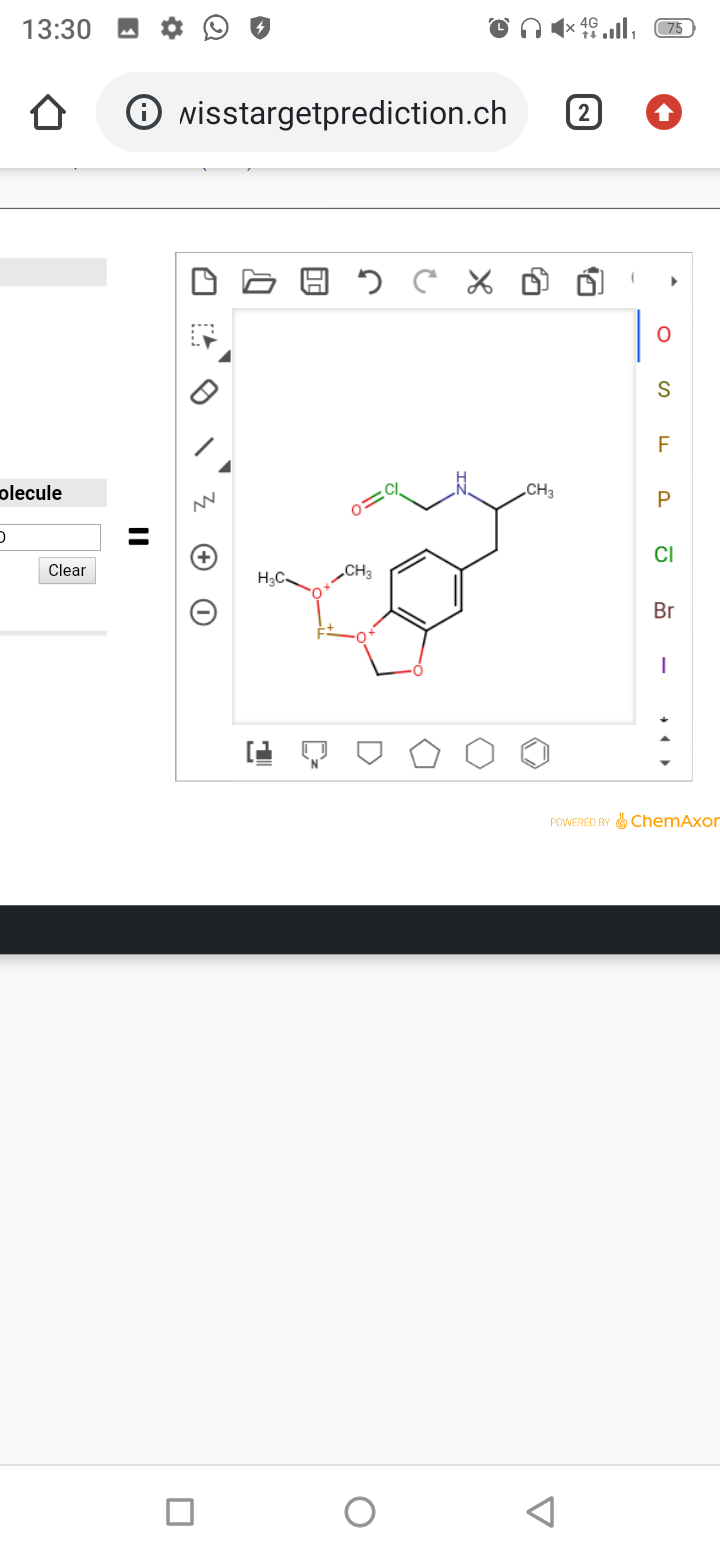
For a few months ago I am developing something I called MDMAv2. Practically, both for personal problems that lead to me to eat antidepressants every day both for pure fun, I started searching for a way to develop a technique to have the effects of the MD we all know without side effects and without incurring problems such as tolerance , not even with chronic intake.
I state that I have no degree, but for a year I dedicate my day of my day to biotechnology and, as I had already said, it has been a few months since I am dedicated to this new project.
The project does not consist of a new molecule, but in developing genetic therapy that goes to add receptors created in the laboratory called DREADDs to neurons of interest. These receptors were designed to activate to molecules that would otherwise be inert as the CNO. For example, adding them only in neurons and excluding heart cells that express the serotonine receptor 2b will not have the cardiotoxicity that has MDMA and avoiding to express them in neuroendocrine neurons are avoided things like teeth grinding. Or again, avoiding expressing them where serotonergic autoreceptors are expressed will avoid negative feedbacks and therefore tolerance and down of the following days. If the normal functioning of the human body are merged, it will remain unchanged since they are used only to drug use.
So the assumption is very simple, we do the injections of genetic therapy to add these dreadds only in the neurons of interest and then when you want to feel the effects just choose one of the many existing molecules on the market to activate them.
However, here is this nice thing I wanted to share with you. If someone wants to help me, I'd love to!
I state that I have no degree, but for a year I dedicate my day of my day to biotechnology and, as I had already said, it has been a few months since I am dedicated to this new project.
The project does not consist of a new molecule, but in developing genetic therapy that goes to add receptors created in the laboratory called DREADDs to neurons of interest. These receptors were designed to activate to molecules that would otherwise be inert as the CNO. For example, adding them only in neurons and excluding heart cells that express the serotonine receptor 2b will not have the cardiotoxicity that has MDMA and avoiding to express them in neuroendocrine neurons are avoided things like teeth grinding. Or again, avoiding expressing them where serotonergic autoreceptors are expressed will avoid negative feedbacks and therefore tolerance and down of the following days. If the normal functioning of the human body are merged, it will remain unchanged since they are used only to drug use.
So the assumption is very simple, we do the injections of genetic therapy to add these dreadds only in the neurons of interest and then when you want to feel the effects just choose one of the many existing molecules on the market to activate them.
However, here is this nice thing I wanted to share with you. If someone wants to help me, I'd love to!
Last edited: