How DMT switches the brain’s reality channel
by Dr. Andrew Gallimore | KAHPI | 12 Feb 2020
Dr Gallimore explains how the sober brain perceives a 'default reality' and how on DMT it seemingly attunes to a completely different frequency.
When I was a child, my parents kept a tiny 1970s black-and-white TV set in the kitchen, presumably relegated from the living room after we went full technicolor. In contrast to the shiny buttons of our swish 1980s color set, this vintage one employed a circular dial to move between channels. Playing with this dial as a curious child revealed the distinct phases of the channel switch: the crisp black-and-white moving image would first distort as the dial was rotated and interference patterns would encroach. This was followed by a complete breakdown of the image into pure noise with no discernible structure. However, continued rotation of the dial would eventually reveal an entirely new channel which would crackle into view: order gave way to disorder which then gave way to a new order. A couple of decades before our little TV set was built, Hungarian physician Dr. Stephen Szara also discovered a channel switch that seemed to operate in a similar way. Self-experimenting with the newly-identified natural psychedelic molecule, DMT, Szara discovered a channel switch for reality itself.
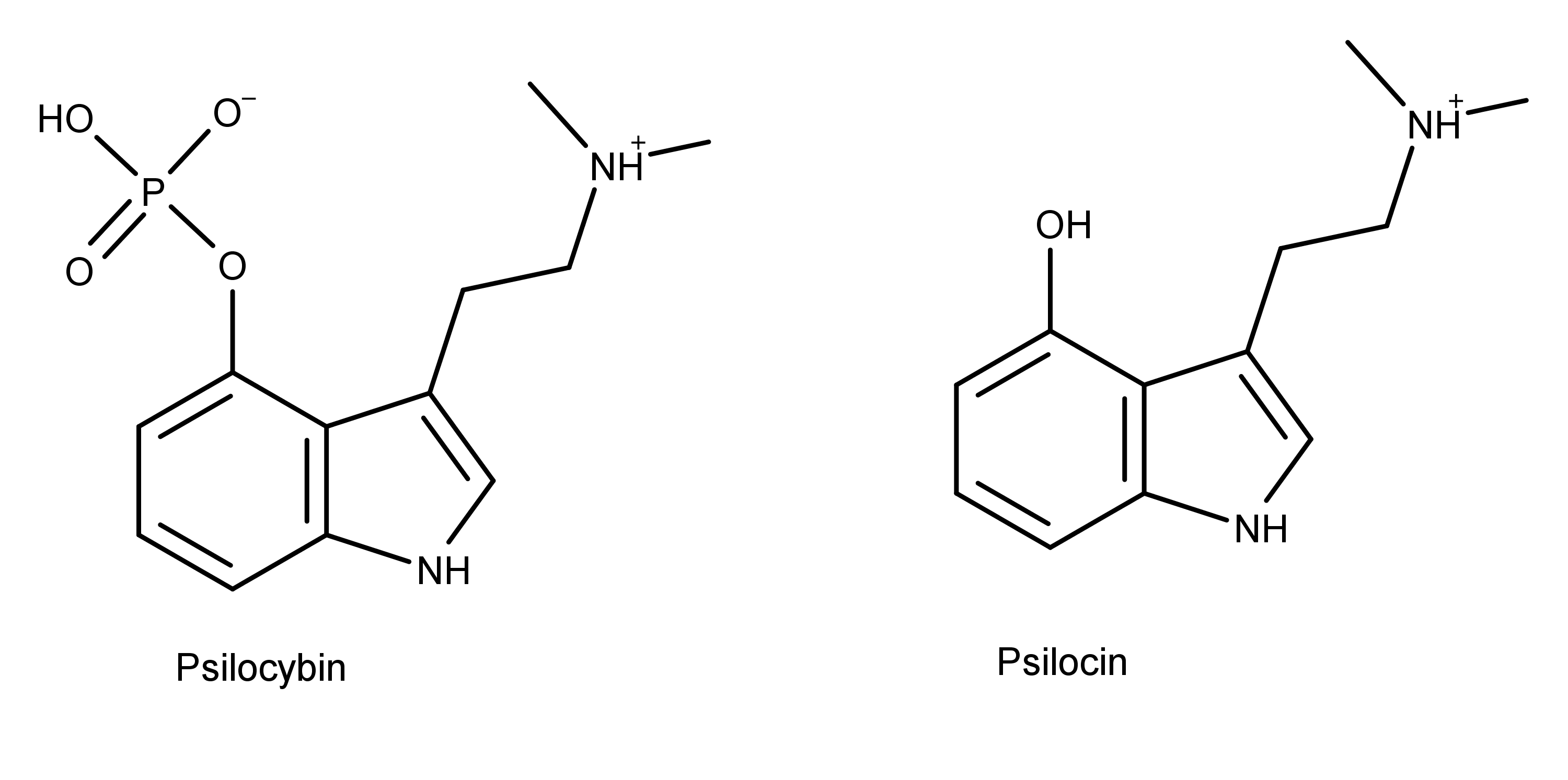
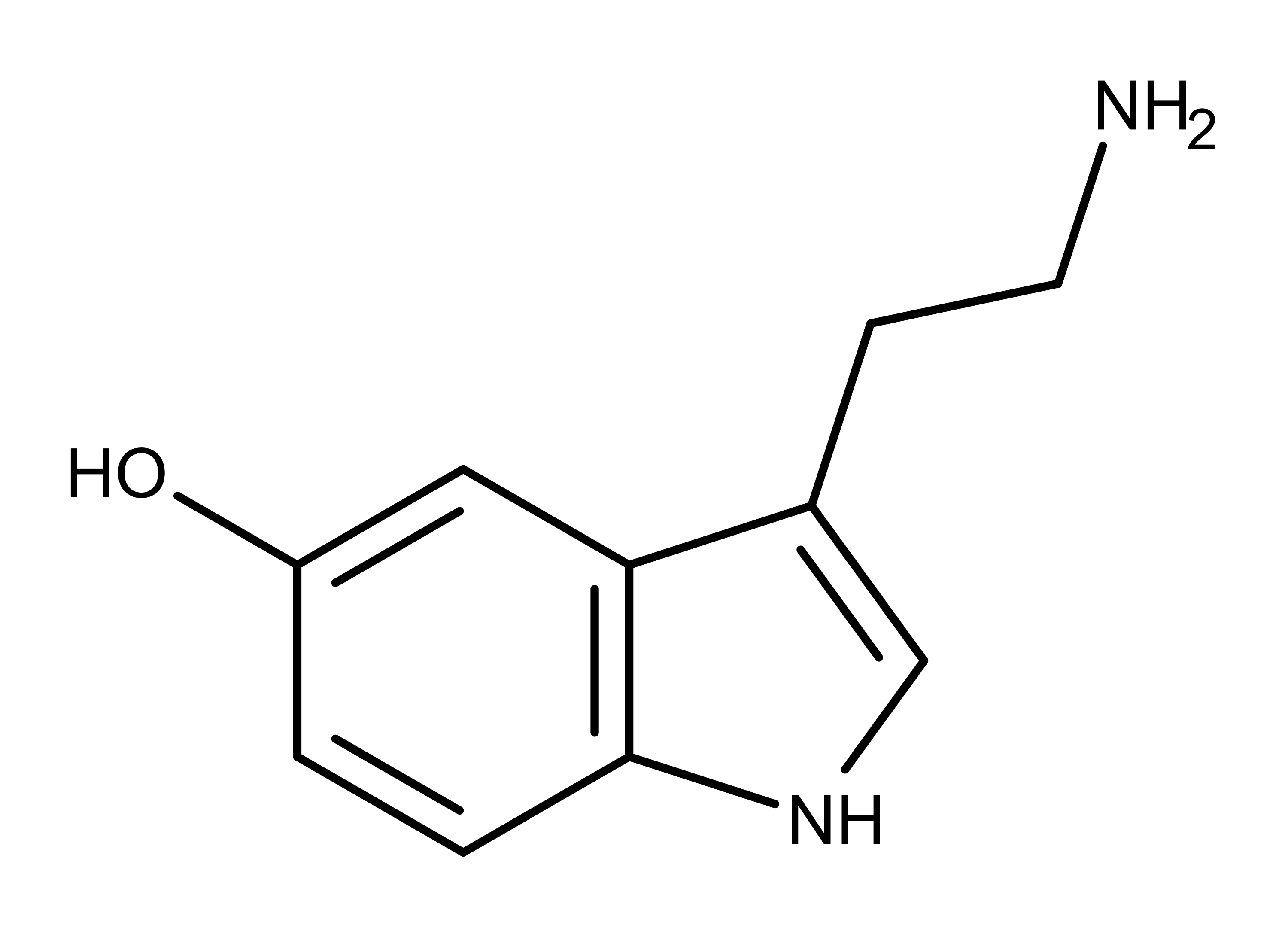
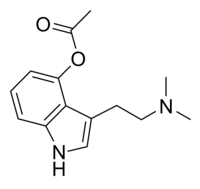
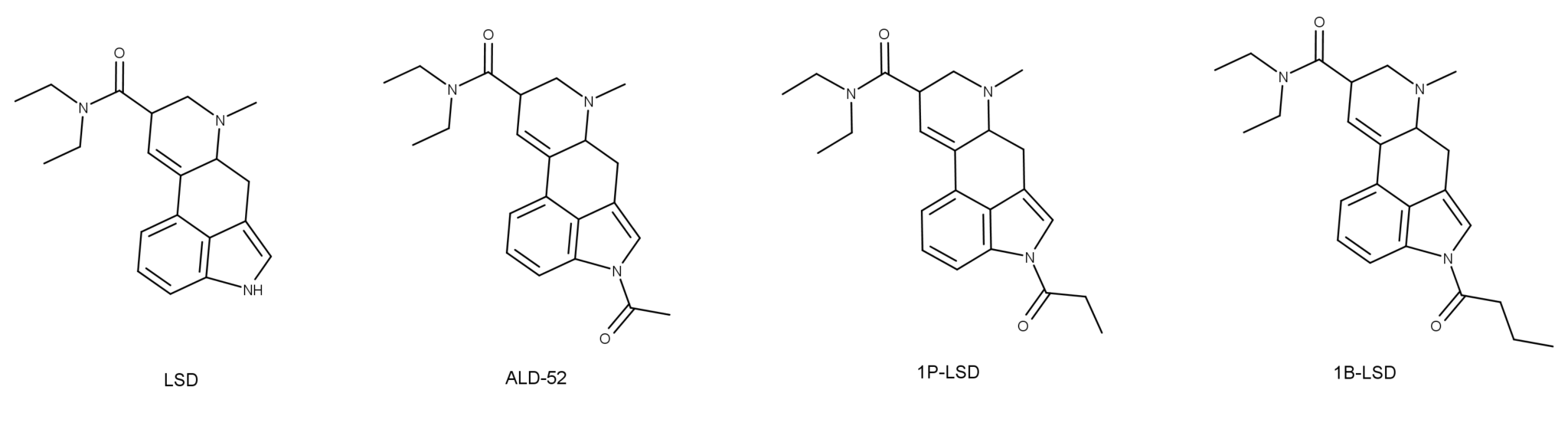
Referring to DMT — N,N-dimethyltryptamine — as a “100 percent reality channel switch,” Terence McKenna perfectly captured what it’s like to be catapulted into the bizarre realms to which this most mysterious of psychedelic substances grants access: after two or three lungfuls of its curious tasting vapor, the old familiar world begins to distort and break down, as complex geometric forms at first veneer the world and then replace it entirely. The tripper is then propelled through a procession of wild and chaotic imagery before finally, assuming the dosage is sufficient, bursting through a veil into an entirely new, astonishingly strange, world: the DMT space.
Although the superficial parallels with flicking to a new TV channel are obvious, a reality channel switch is actually a rather appropriate descriptor for the effects of DMT on brain activity during a breakthrough trip. To understand how this switch works, we first need to consider how the brain, in the absence of DMT, constructs and maintains the channel of your normal waking world.
Your brain on Channel Consensus Reality
To be born is to be born into a world. To be conscious is to be conscious of a world. Whether you are awake, dreaming, or at the peak of a psychedelic experience, you are always immersed in a world. Of course, the extremely bizarre world of high-dose DMT bears little resemblance to the normal waking world — often referred to as the consensus world — whereas the dream world is usually much more familiar. And, of course, there are other types of worlds that can be distinguished: the fragmented worlds of a schizophrenic patient or the utterly ridiculous and often horrifying worlds that are experienced after taking concentrated Salvia divinorum extracts. But what unites these disparate worlds is their subjectivity: whenever you are conscious, your world is your own unique subjective world experienced from behind your eyes, the world you live in, and the only world you will ever know. While the structure of that world might change dramatically depending on your state, your subjective, phenomenal world is always yours and yours alone.
The world you live in during most of your waking life is clearly your default world, and we can turn to the author Hermann Hesse for a beautiful articulation of what this subjective world actually is:
“If the outside world fell in ruins, one of us would be capable of building it up again, for mountain and stream, tree and leaf, root and blossom, all that is shaped by nature lies modelled in us.”
Intuited by Hesse over 100 years ago, your normal waking world is, in more modern scientific parlance, a model of the environment built by your brain. The purpose of this model is to provide you with what philosopher Thomas Metzinger calls a “simulational space” that you can use to navigate and interact with your environment. Obviously there’s a relationship or a mapping between the model and the environment itself, but the world you experience is always this model. When you smoke a sufficient dose of DMT, what changes, in a shockingly dramatic manner, is this model constructed by your brain.
The human brain is obviously an exquisitely complex machine that appears almost magical in its ability to accomplish a bewildering array of complex tasks required for life. But, in truth, the brain really has only a single, albeit extremely complicated, job: to receive, process, and generate information. This information is generated by specialised cells called neurons which fire electrochemical signals that can be passed to other neurons via specialised chemical connections called synapses. The strength of these chemical connections can be increased or decreased to control the flow of information between neurons. The outer layer of your brain is known as the cerebral cortex. It is responsible for building your world model and is essentially a folded sheet containing billions of these interconnected neurons. Closely connected groups of neurons form cortical areas specialised and tuned to receive, process, and generate particular types of information that correspond to particular features of the world, such as lines, colours, textures, spatial relationships, and specific types of objects.
The cortical areas are connected, using synapses, to form networks that control the structure and flow of information through the brain, allowing it to “sculpt” its model of the world. By modifying these patterns of connections, throughout the course of evolution, development, and experience, the brain improves and refines this model. The phenomenal world you experience from moment to moment is the highly complex pattern of information sculpted by these networks of brain areas. Or, equivalently,
your phenomenal world is what this pattern of information feels like from your subjective perspective. Your world is built from information.
Of course, this model would be perfectly useless if it didn’t allow you to navigate your environment, to avoid dangers and locate food, to catch the eye of a potential mate and, more generally, to make judicious decisions (“Is that a snake or a coil of rope?”) and predictions (“Is this car going to hit me?”). In other words, the model must be stably tuned in some way to the environment. The brain achieves this tuning by constantly testing its model against incoming sensory information (from the eyes, ears, etc). Essentially, the brain uses the model to predict the patterns of sensory information entering the brain from moment to moment (“If the model I’m using is good, what should I expect to happen next?”). If the prediction is successful, then that sensory information is suppressed, meaning it isn’t passed into the networks of the brain for further processing.
Since neurons use energy, information processing is expensive, so it doesn’t make sense for the brain to process sensory information that’s already part of the model because, in a way, that information is already known. However, if unexpected and surprising patterns of sensory information are received — if the model prediction is incorrect — then error signals are generated, which are then passed into the cortical networks and used to update the model to reduce those error signals.
So, the brain doesn’t need to build its model from scratch from moment to moment, but merely to update it by focusing on unpredictable, surprising information, and filtering out predictable sensory information that fits the model. This constant, real-time, testing and updating against sensory information allows your brain to tune into the environment, locking you into what we might call Channel Consensus Reality.
The robustness of this consensus reality model reveals itself as you descend into REM sleep at night and begin dreaming. It’s no coincidence that most dreams resemble the normal waking world in pretty much all aspects. In fact, dreaming is largely continuous with waking and studies have shown that the proportion of time you spend performing mundane daily activities, such as watching TV or talking on the telephone, is similar in the dream world as it is in the waking world. The crucial difference is that, during dream sleep, your brain is disconnected from sensory information. This renders the dream world familiar and yet distinctly erratic and unstable. The brain is able to rearrange the pieces of its model into unusual, often impossible, ways during a dream, since it isn’t constrained by having to constantly test the model against sensory information. The dream world is thus much more fluid and unpredictable than the normal waking world, despite being an unmistakable variation of it. The similarity of the dreamscape to Channel Consensus Reality isn’t that surprising, since the brain is using the same model to construct the dream world as it does to construct the waking world. What’s more surprising is what happens to the waking world when certain psychoactive molecules enter the brain.
Your brain on psychedelics
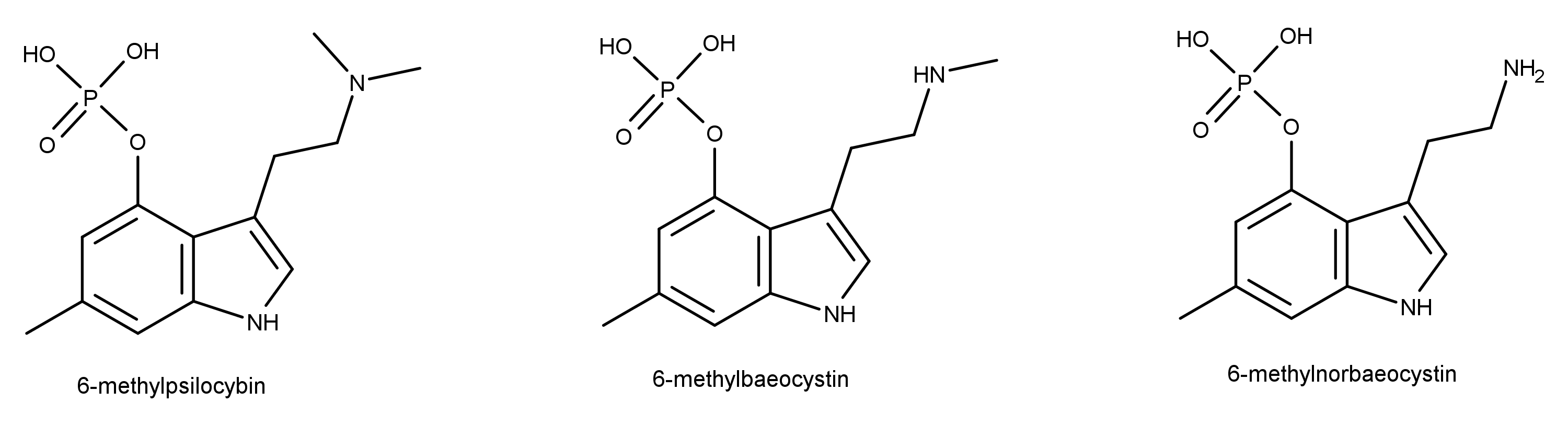
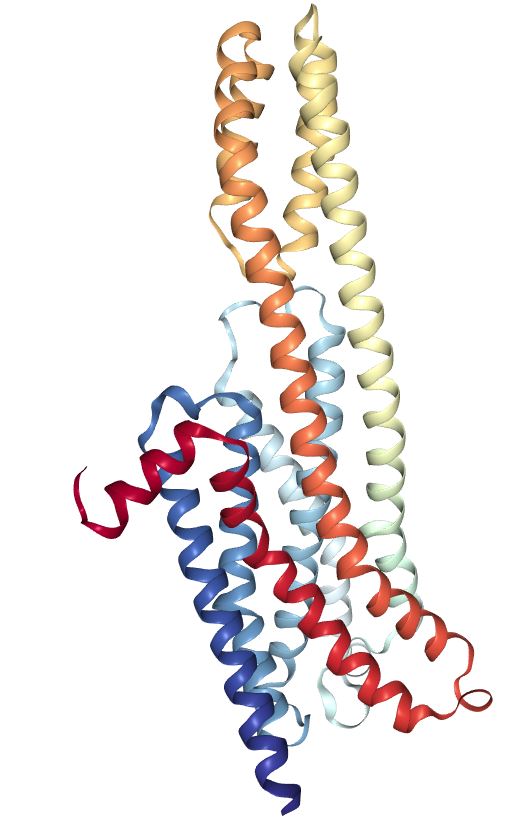
Although psychedelic plants and fungi have been used by indigenous cultures across the globe for thousands of years, it’s only in the last century that they’ve become firmly rooted in Western culture. The so-called classic psychedelics include LSD, psilocybin (from “magic mushrooms”), mescaline (from the peyote cactus), and DMT, in addition to a range of chemically related molecules, and are unified by their characteristic mechanism of action in the brain. The classic psychedelics exert their effects by binding to a specific type of serotonin receptor — the 5HT2A receptor — that is heavily expressed in layers of the cortex with a crucial role in constructing your world model. Upon binding to the 5HT2A receptor, these psychedelics stimulate the neurons in these layers, making them much more responsive to incoming signals from other neurons and much more likely to fire signals that are then passed to other neurons via their synaptic connections. The overall result is a highly excitable layer of neurons over large areas of the cortex, with information flowing much more freely between cortical areas. Since the brain’s ability to construct a coherent model of the environment relies on a delicately constructed pattern of weighted connections between brain areas, this information free-for-all disrupts or “shakes up” the world model.
When research volunteers are given a psychedelic and placed in an MRI machine, functional imaging scans reveal the distinct patterns of brain activity induced by these substances. In the case of both LSD and psilocybin, normally well-ordered patterns of brain activity are seen to break down as information begins to flow out of formerly well-demarcated networks, and networks that were once disconnected begin speaking to each other.
This is what leading psychedelic researcher Dr. Robin Carhart-Harris calls an “entropic” (i.e. disordered) or “hot” state. From the perspective of the psychedelic user, the world undergoes a distinct shift from being stable and predictable to being unstable, fluid, and unpredictable. The brain’s model of the world begins to fall apart. Naturally, this fragmented model becomes less successful at predicting sensory information, which leads to an increase in error signals flowing through the networks of the cortex. Recall that the brain filters out the sensory information it correctly predicts, with only the unpredicted and surprising information being processed in the form of error signals. By disrupting the brain’s ability to predict sensory information, psychedelics effectively remove this filter and information that would normally be filtered out suddenly fills the world and everything becomes surprising, salient, and novel. Anyone who’s ever taken either LSD or Psilocybe “magic” mushrooms will know the subjective effect of this neurological shift: colours become brighter and more pronounced, objects pop out of the surroundings and become imbued with profound significance and meaning. Everything becomes deeply fascinating, as the entire world is seen anew, as a child.
In short, under the influence of a psychedelic substance, the brain loses its grip on the flow of information both into and through itself. And, naturally, it attempts to correct the situation by updating its model — as it would under normal circumstances when error signals are generated — but it’s unable to form a stable and coherent model which reduces the error signals. As such, it continues searching, updating, and the subjective world becomes unstable, shifting rapidly in response to the unfiltered barrage of information from the environment.
This is clearly observed in the MRI scanner, as the brain seems to move in a disorderly fashion through an expanded number of different patterns of neural activity, many of which are only observed under the influence of a psychedelic. It’s as if the brain has been nudged out of tune and is fumbling with the dial attempting to retune itself. From the tripper’s perspective, the world becomes fluid and unstable: everything moves and flows, objects merge or change their identity from moment to moment, the garden hose on the lawn becomes a coiled snake, the pebbled driveway a bed of gleaming jewels.
DMT: The reality channel switch
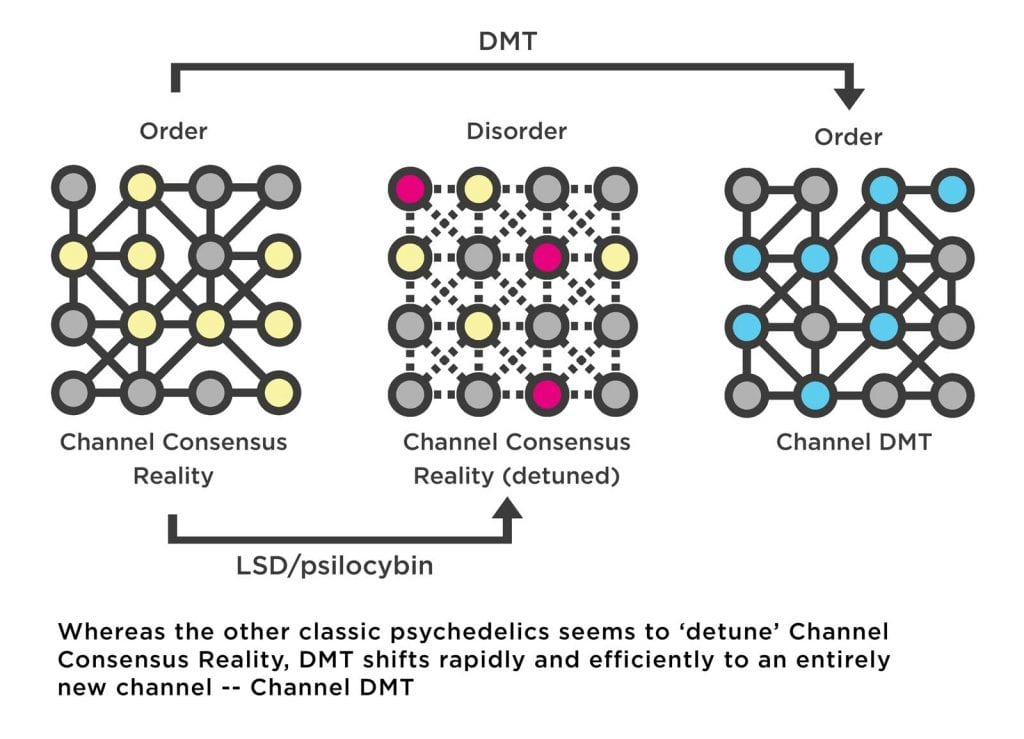
Although DMT also acts mainly via the 5HT2A receptor, its effects upon flooding the brain are dramatically different to regular doses of the other classic psychedelics: rather than simply being nudged out of tune, it’s as if the brain has been switched to an entirely different channel. The brain reaches for the dial and, with effortless precision, shifts to the new frequency. From a more neurological perspective, the brain ceases to construct the normal waking world model and begins to construct an entirely new model, which is experienced as an entirely new world.
Assuming the dosage is sufficient, the breakthrough DMT state doesn’t present itself as a maelstrom of confusion and chaos — although this does typify the early stages of the experience — but rather fully realised worlds of crystalline clarity and replete with a diverse ecology of intelligent beings eager to communicate with the tripper. The switch from Channel Consensus Reality to Channel DMT is swift, efficient, and complete.
While there are still relatively few neuroimaging studies of the DMT state in humans, this data gap is now being filled by the pioneering Centre for Psychedelic Research at Imperial College London (among others), which recently published a detailed study of the effects of DMT on neural oscillations measured using EEG. Neurons are electrochemically active cells and, when very large numbers of these cells are connected and active — as in a human brain — electrical oscillations of various frequencies emerge, synchronise, and propagate across the cortex, and can be detected through the skull. Different frequency bands have particular roles in brain function, and synchronisation of oscillations can help in organising the structure and timing of neural activity. In particular, so-called alpha-oscillations are associated with the brain’s ability to model the world and predict patterns of sensory information. Studies with LSD and psilocybin reliably show a decrease in the strength of these alpha oscillations, as well as desynchronisation of several types of oscillations, indicating the disruption of the brain’s world model and the increase in disorderly patterns of neural activity. However, whilst these effects were also observed in the DMT study — since DMT also acts mainly at the 5HT2A receptor — the researchers also recorded a surprising increase in the strength and synchrony of lower frequency oscillations known as delta and theta oscillations. They described this novel effect as the emergence of “apparent order amidst a background of disorder.” These delta/theta oscillations are normally associated with the dream state, when the sleeper is immersed in a world disconnected from the environment. However, while the dream world is built from the same model as the waking world (but without sensory testing), the DMT world is utterly incomparable and must be constructed using an entirely different model. As such, it’s likely that this new order that emerges during the DMT state indicates that the brain has successfully found a stable new channel — Channel DMT.
Of course, switching to Channel DMT doesn’t necessarily mean that the brain has actually tuned to a freestanding alternate reality populated by conscious intelligent entities. Like all phenomenal worlds, including the waking world and the dream world, the DMT world is constructed by the brain — it is a model built from neural information. This would be the case whether Channel DMT is receiving information from, and tuned to, a normally hidden and inaccessible external environment (as with Channel Consensus Reality) or whether it is constructed while disconnected from sensory information (as with the dream world). Obviously, most scientists would favor the latter explanation, since few would entertain the idea that DMT is somehow gating access to a normally hidden parallel world bursting with wildly giggling elves. However, this orthodox explanation isn’t without its problems.
When the brain switches to Channel DMT, it begins constructing a model of an environment that is entirely unrelated to Channel Consensus Reality. This world model possesses structures, content, and qualities that are characteristically “DMT-esque” and appear common to large numbers of independent users around the globe: a number of entities — including the ubiquitous “machine elves” — as well as the bizarre hypergeometric and curiously technological rooms, temples, and landscapes they occupy have come to typify the DMT state.
Spend some time trawling through the online DMT trip report literature and one can’t help feeling that many DMT users often end up being fired into the same place and meeting the same entities. From an orthodox neuroscientific perspective, this is confounding. As far as we are aware, the brain ought to know how to build only a single type of world model: the old familiar consensus world. This is the world — the interface with the environment — the brain evolved to build and the world it continues to construct even during dreaming. As such, the brain’s ability to suddenly begin constructing a bizarre alien world model bearing no relationship whatsoever to the normal waking world is as confounding as a 5-year-old British child suddenly switching to speaking fluent Central Siberian Yupik. Or, one might compare it to finding an entirely new channel on your TV set and then discovering that the aerial has been disconnected. Where did this bizarre world come from? How did the brain learn to construct a model of it? Of course, it would be much easier to explain this if, as countless DMT users are convinced, Channel DMT is tuned to — receiving and processing information from — an alternate reality existing independently of our brains and, most likely, outside of our Universe. But, of course, as professional and sofa scientists alike are always keen to sneer: that’s impossible.
Confronting DMT means confronting the truly impossible. Nothing about this substance seems to make sense. From inside the trip, as you’re hurled screaming through those luminal corridors of ineffable complexity and beauty, or sat at the feet of beings of unreckonable intelligence and power, it all seems perfectly impossible. And from the outside, from the perspective of scientists and philosophers trying to make sense of this substance, an explanation seems no easier to come by. When Terence McKenna first stumbled across Channel DMT in his tiny Berkeley apartment in the Fall of 1965, he admitted to having never gotten over it. And, to be honest, I’m not convinced I ever will either. It just doesn’t seem possible.
Dr Gallimore takes us through how the sober brain perceives the default reality and how DMT in the brain makes it attune to a wholly different frequency.

kahpi.net