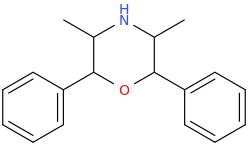
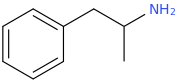
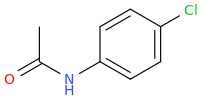
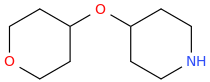
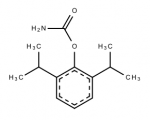
Dresden
Bluelighter
- Joined
- Feb 2, 2010
- Messages
- 3,212
^--Nice.
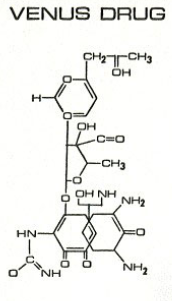
Here's Another One:
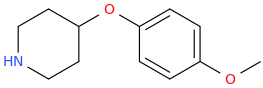
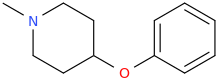
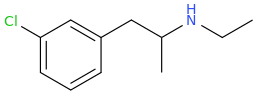
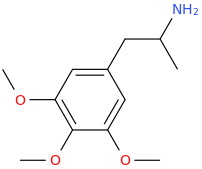
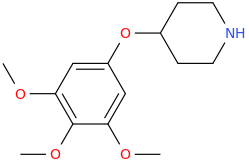
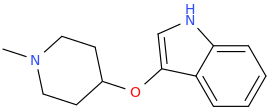
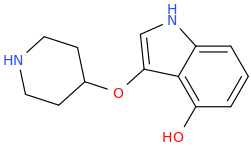
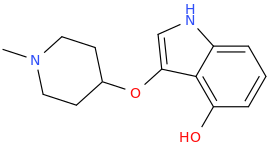
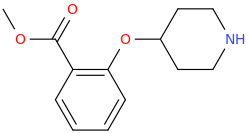
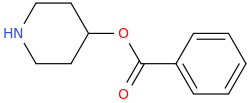
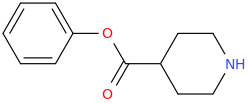
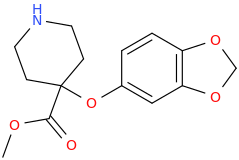
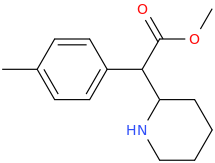
L.L. [LARRY LILAC] BEAN
(Lilacs contain 1,4-dimethoxybenzene, which I conjugated with piperidine and gave a creative name to.)
I'd Try It!!!
I would rather try Disco Crisco, though.
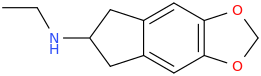
KIMBERLY
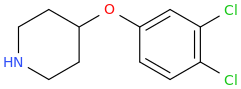
AHNOLD, ideal for fighting or race car driving.
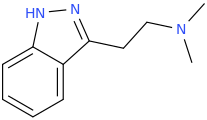
DAVID

TINKY WINKY, a Teletubby.
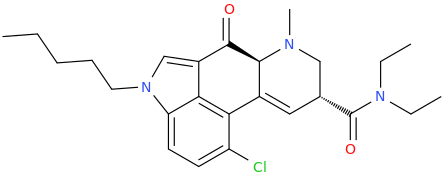
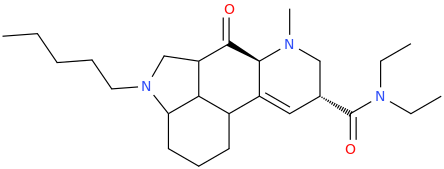
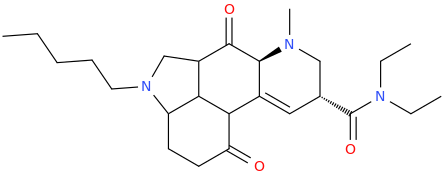
'JWH'-2019
Has this one been thought of lately? Seems like an obvious MXE congener of sorts to me.
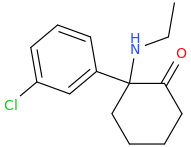
MISTER PEABODY
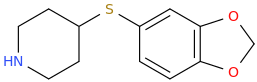
SWEET 16
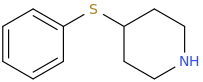
CRUNK MUFFIN
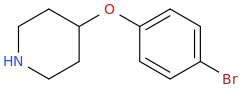
BLADE RUNNER
And There Ain't No Road Just Like It Anywhere I've Found, Blade Running South On Lake Shore Drive Headed Into Town. And It's 4 AM In The Morning, And All The People Have Gone To Sleep. It's Just You And Your Mind And Lake Shore Drive. And Tomorrow Is Another Day. And The Sun Shines Bright In The Morning Time. And Tomorrow Is Another Day.
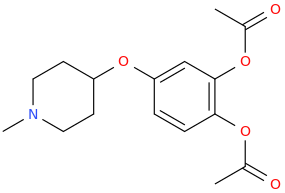
RON 'OPIE' HOWARD
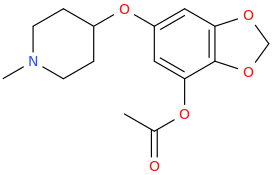
AL 'VISHNU' BUNDY
Love Is Blind.
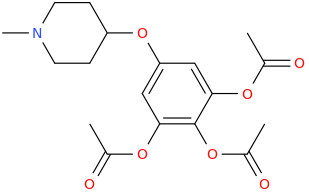
LORD HARLEY 'SO SO DEF' DAVIDSON
The Fear Of The Lord Is The Root Of Righteousness.
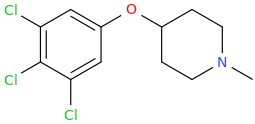
ANDREW 'SHIVA' CHRISTIAN
My 'Dance' Keeps Every Universe Going And Is My Duty.
YOU NEED CANDY.
C ANDY
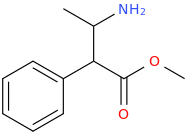
ANDY
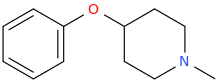
GOMER PILE, suspected opiate.
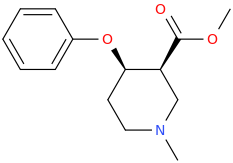
SHAZAAM
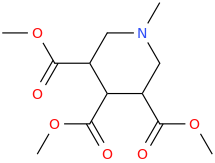
CICCONE
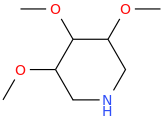
HI CHEW
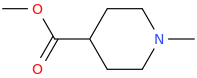
PIKACHU
I would be kind of surprised if these last few did much of anything, but hey, I've been wrong before.
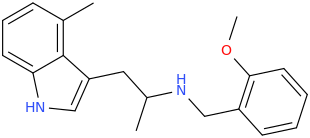
FormerBeagle
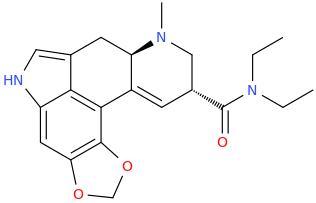
Go Ask
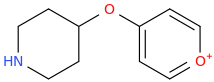
ALICE In Wonderland.
Note: I realize pyrylium salts are not usually stable, but ALICE and her congeners are intended to be amine salts.
Here's Another One:

L.L. [LARRY LILAC] BEAN
(Lilacs contain 1,4-dimethoxybenzene, which I conjugated with piperidine and gave a creative name to.)
I'd Try It!!!
I would rather try Disco Crisco, though.

KIMBERLY

AHNOLD, ideal for fighting or race car driving.

DAVID

TINKY WINKY, a Teletubby.

'JWH'-2019
Has this one been thought of lately? Seems like an obvious MXE congener of sorts to me.

MISTER PEABODY

SWEET 16

CRUNK MUFFIN

BLADE RUNNER
And There Ain't No Road Just Like It Anywhere I've Found, Blade Running South On Lake Shore Drive Headed Into Town. And It's 4 AM In The Morning, And All The People Have Gone To Sleep. It's Just You And Your Mind And Lake Shore Drive. And Tomorrow Is Another Day. And The Sun Shines Bright In The Morning Time. And Tomorrow Is Another Day.

RON 'OPIE' HOWARD

AL 'VISHNU' BUNDY
Love Is Blind.

LORD HARLEY 'SO SO DEF' DAVIDSON
The Fear Of The Lord Is The Root Of Righteousness.

ANDREW 'SHIVA' CHRISTIAN
My 'Dance' Keeps Every Universe Going And Is My Duty.
YOU NEED CANDY.
C ANDY

ANDY

GOMER PILE, suspected opiate.

SHAZAAM

CICCONE

HI CHEW

PIKACHU
I would be kind of surprised if these last few did much of anything, but hey, I've been wrong before.

FormerBeagle
Go Ask

ALICE In Wonderland.
Note: I realize pyrylium salts are not usually stable, but ALICE and her congeners are intended to be amine salts.
Last edited:


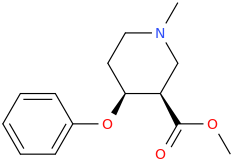
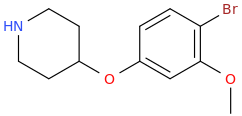
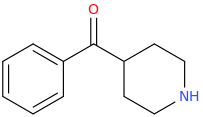
![2-[(dimethylaminocarbonyl)oxy]-N,N-dimethylpropanamine.png](/community/proxy.php?image=https%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F2-%5B%28dimethylaminocarbonyl%29oxy%5D-N%2CN-dimethylpropanamine.png&hash=544d30ef6a1c533ade7c01ffa6561cd7)