haribo1
Ex-Bluelighter
- Joined
- Nov 29, 2006
- Messages
- 4,826

Phenmetrazine is said by many old, old, incredably ooolllld school speed freaks that phenmetrazine was a far superoir drug to benzedrene or dexedrine. I know in a book called 'Rush' a woman describes shooting it as 'the most prosexual drug ever'.... although needles would sure dampen my ardour.
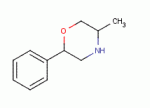
Well, I've been trying to figure out the SAR for the aminorex series (with and without the 4 methyl).
Of course, then I thought... 'what will happen if you take a methyl off then end pf phenmetrazine?
The ring will pull the amine into some kind of active shape. I know people have tried substituting both aminorex, 4 methyl aminorex and pemoline but has anyone considered the morpholine ring?
Where would this stand on the UK MoDA laws? I would feel confident to try it out, I don't think that it's basic structure is known to be dangerous. Maybe the 2,5 dimethoxy 4 <something> would make a good starting point. Make say 1Og, seperate all the isomers and work up form 250ug (just like Hoffman).
If this has been tried and I missed it, and sorry. If not, has anyone got the patent on making phenmetrazine? I know it will be pretty trivial to make it, but the patents offer some kind of framework....