Microdosing ibogaine for Parkinson's
by Lakshmi Narayan and Eric Swenson | Awake.net
Patient D is a 76-year old individual who was diagnosed with Parkinson’s five years ago and has been able to manage and improve his symptoms by microdosing ibogaine. He remains anonymous because of the legal status of ibogaine. Ibogaine is an entheogenic plant medicine that has helped him to have a semblance of health and vitality even with such a crippling disease as Parkinson’s. We spoke with Patient D about his experiences.
LN: How old were you when you were diagnosed with Parkinson’s?
Patient D: I’m 76 now, I was 71 when I was diagnosed with Parkinson’s.
ES: And I understand that your doctor diagnosed you with “atypical” Parkinson’s. What was atypical about it, beyond the fact that you had no tremors?
Patient D: That’s all. In fact, most of the people with Parkinson’s don’t have tremors.
ES: Oh, I didn’t know that. I thought that was pretty standard.
Patient D: Yeah. I probably know more about Parkinson’s than most doctors. I’m an authority on the subject because I can put words to what I experienced and they can’t. They don’t know what I’m talking about with Parkinson’s.
ES: Right. People have told me that with Parkinson’s everything is diminished.
Patient D: That’s it. Everything.
ES: What is your ability to smell? That is another issue for many Parkinson’s sufferers.
Patient D: Smell and taste have been coming back very slowly. They disappeared completely. But now I can smell, I can taste. It was gone for a long time. I now sense more improvement rather than more problems happening with my Parkinson’s.
I’m a member of the local Parkinson’s group. There are about 150 people that come once a month. And it’s quite an education. I can’t talk to anybody about what I’m doing, because I used to–and they all got very paranoid.
They’d say,
“Well, how do you spell that?” And I spelled it for them.
I-B-O-G-A-I-N-E
When they look on the web, all they can find is, it’s about heroin addiction. It’s used for heroin. It just freaked them out. They didn’t want to have anything to do with it.
LN: I see. Well, that’s very enlightening, because it’s as though ibogaine, the medicine itself, has been stigmatized by association.
Patient D: That’s right. So I have not had one person that I’ve talked to at the local group that wants to do it. Not one. I did a lecture in Mexico four or five years ago, and there were a couple people at that conference who were doing it because of what had happened to me a year before, when I took it for the first time. And they’re still taking it, and they’re still just about the same as I am.
LN: So could you describe what happened when you took it for the first time?
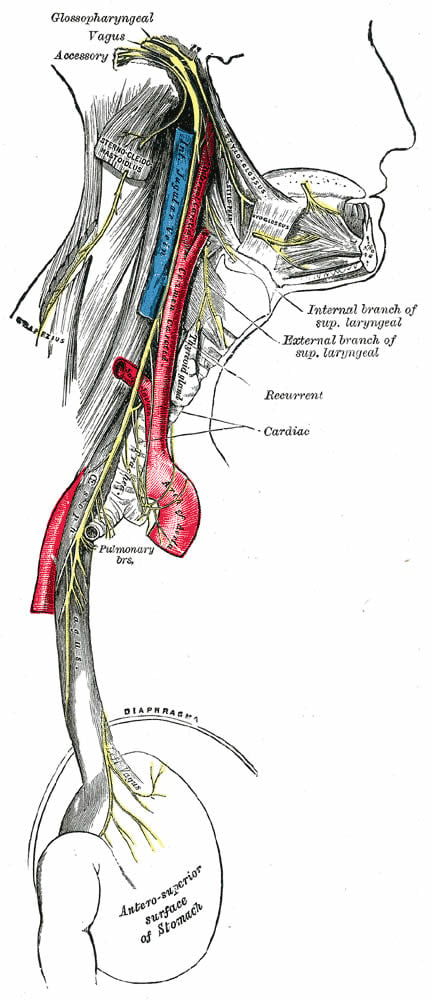
Patient D: I started taking it in November of 2014. I heard about it from some people in New York, ex-heroin addicts, who believed that because Parkinson’s affects dopamine, they had found that ibogaine helps the creation of dopamine through providing GDNF. It’s a neuronal approach to it. GDNF helps the neurons grow and secrete dopamine, so they thought maybe it will increase the flow of dopamine in Parkinson’s people, from another place, a different place than the substantia nigra, which is that little teeny piece in the brain that creates it.

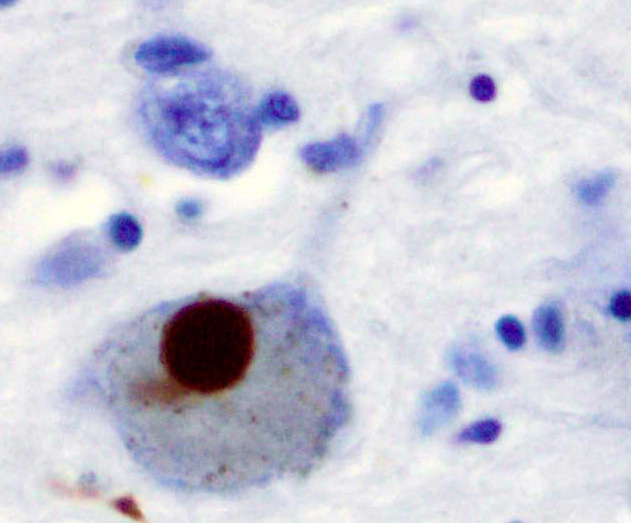
Parkinson’s disease (PD) is a neurodegenerative disorder that affects predominantly dopamine-producing (“dopaminergic”) neurons in a specific area of the brain called substantia nigra. Parkinson’s symptoms generally develop slowly over years. The progression of symptoms is often a bit different from one person to another due to the diversity of the disease. The cause remains largely unknown. People with PD may experience: tremor, bradykinesia, limb rigidity, gait and balance problems.
LN: What made you decide to take Ibogaine when nobody else had taken it for Parkinson’s— what did you do, how did you find it, where did you go, what did it do for you afterwards?
Patient D: I’m it. I mean, I really am. I’m the guinea pig. And, it’s all sort of anecdotal evidence. There’s no scientific rigor applied here. So you can’t really use me as a scientific study. Although I can tell you from first-hand experience that this stuff, ibogaine, it’s incredible. And these people are silly for not wanting to do it, but they associate it with crime and drugs. And I don’t want to be associated with that, in their eyes, either. Because I’ve been down once. I don’t want to go down again.
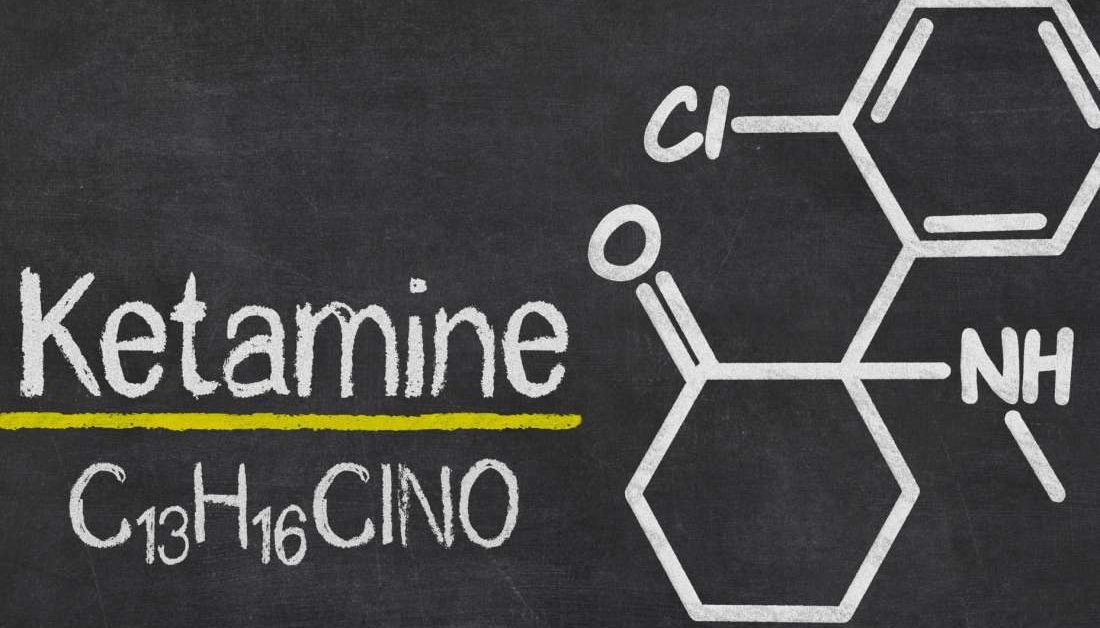
So what happened to me was, I had some friends that were friends with these people in New York that had access to ibogaine, and one of them had used it to cure his heroin addiction. He’s now probably 75. He thought that this was going to be a business for him, making it available for Parkinson’s patients, and he gave me enough to use — he had it pre-packaged in very small microdose amounts. I started out taking four milligrams, twice a day, for a week, and then I did that for two weeks.
And even at four milligrams, which is a very small amount, after two weeks I had VAST improvements.
All my joints used to ratchet, they’d go click, click click click click, when I’d try to go through a movement. And now they’re smooth; I can stand up now, and walk down a straight path, and do a U-turn, and come right back without a problem. Parkinson’s people, it takes them three or four steps to make a U-turn. That was me before I started this, I was doing that. You freeze in place, where you can’t move. You have to struggle to get your body to want to follow your orders. So I felt really, when I discovered that I had it, that I had a death sentence imposed on me. Parkinson’s is a death sentence. And I don’t want to die. I’m not ready to die yet. I’ve got young kids, relatively young, now, I’m considering. So, I said sure I’ll take that stuff. I wasn’t afraid of psychedelics. I did and it worked a bit, and it keeps working. I’m very happy.
ES: So I’m interested in the dosage. It is what microdosers call “sub-perceptual,” correct? You do not have a psychedelic effect taking a microdose?
Patient D: You’re correct. I do not have a psychedelic effect. I started out at eight milligrams a day. And after a month I was taking twice and then three times that. “This is working. I should keep trying more!” Which is, I guess, the age-old “more is better” concept. I ended up taking 40 milligrams, twice a day. 80 milligrams. It’s not very much. Except my friend in Canada is doing 20 milligrams, twice a day. And he’s had the same effect. So I’m gonna try and take less. See what happens. And I’ve tried to take less before, and it didn’t work. I have to take this much to maintain where I’m at.
LN: So the other people who took it, did they also feel better?
Patient D: Oh yeah. One guy is a carpenter, and he had to quit working because of his Parkinson’s. He’s 55 and now he’s working and building a house in Canada. His body is working again. He rides a bicycle. The key also, besides Ibogaine, the other key, is a lot of exercise. I can’t ride a bicycle because of my balance condition, but I have a bicycle machine that I strap myself into. And it pedals me. I can resist it, I can decrease pressure on it. But I ride on that and I ride an hour a day.
It’s really a shame that this isn’t available through a prescription through a medical provider, where I could talk more freely to people and let them look at me, let them measure me, let them test me. I’ve had blood tests every year since November of 2014. My annual physical, my blood, has not changed in the slightest amount. So somehow it’s working.
I had a heart echogram yesterday. I’m absolutely normal for somebody my age. A very small amount of thickening of one muscle, for hypertension. I’ve had high blood pressure for 30 years. The only thing I hate about sitting is you get a little bit of a belly. I normally was very thin and active most of my life. Aging is what it is. It’s just some other thing you face.
LN: So you started to take this ibogaine microdose and then you continued to ramp up, and now you’re on a maintenance dose. And that’s like going to be for the rest of your life, you think?
Patient D: If I can maintain my supply, and that’s not easy. It’s the hardest part of the whole game. That’s why I’m so paranoid. I don’t want the risk when having to cross the border. I have to leave this country. I don’t want my face to pop up.
LN: I see.
Patient D: And they can be as vicious as they want to be.
LN: I can see that you would want to be cautious.
Patient D: I was writing a script on marijuana smuggling. I was around people who were smugglers. I was worming my way into their trust. I never actually dealt anything with them. It was all something that was going to happen in the future. But the DEA was monitoring this whole thing. They didn’t know that I was not involved. But when I presented my defense at trial, they didn’t care. I mean, I was tried with two other guys that did do a deal. And I got a 15-year sentence of which I served five. So I don’t want to go back.
LN: It’s a cautionary tale.
Patient D: It was an experience. I don’t regret that I was there. But I don’t want to go back again. It would be too hard at my age. They don’t have any accommodation for disabilities.
LN: Could you go through some of the symptoms you’ve had?
Patient D: Yeah. I know one of the key things that I remember was at some point before I went to Mexico and took this drug as a test. I felt I was losing my edge. I’m a photographer and a writer. And I felt like something had happened to me where I had no edge. I couldn’t think critically. And I hated that.
It was just almost unbearable, to not have the creative side of you active in promoting things. So there was a story in Newsweek, or maybe The New Yorker, about a guy who’d lost his edge. And he was describing me.
Well, after ibogaine, my edge came back! The fact that I can carry on this conversation with you, having full blown Parkinson’s, is a feat in itself. My voice isn’t the same as it always was. My voice has inflection, it has different ranges of emotions. So just be able to communicate.
LN: My mother died of Parkinson’s at age 83. She had it for about 10 years. And during the last few years, she couldn’t do anything. She couldn’t even move. Her hands were like, literally there was no juice in them. There was no electricity, she couldn’t do anything.
Patient D: Dyskinesia. It’s like a fox, he flails around. I can sense it. It’s there. I can feel it within me. But I don’t do it. I’m very fortunate about that.Most Parkinson’s people have a tremor in their hand, their hand just shakes on them, you know, and they always have to stick it in their pocket, or they hide it under a coat. Totally embarrassing, I guess. I have one friend from high school, her husband has Parkinson’s. She’s a wealthy suburban woman who inherited a lot of money, and she’s having to change her lifestyle, from whatever amount of money they can spend each month, because the only drug that works for him, that helps reduce his symptoms, costs $9,000 a month. It’s one that’s a new release; it eliminates some of the symptoms, IF you can afford it.
So, there are no other drugs, except for the classic carbidopa levodopa, which simulates dopamine, but it doesn’t produce dopamine. It just has some effect on your sense of well being and your muscle tone, that calms them down. But other than that, there’s nothing that can help you. They come up with derivatives of this and that, makes me sick. I’ve tried them all. The doctors. I told one doctor what I’ve done and what I’ve experienced. And he says,
“Shut up, I don’t want to hear about it. What am I supposed to do with that information?” It’s true. He'd get himself into trouble.
LN: So, do you take any medications now, other than ibogaine?
Patient D: I take a whole host of medications, for blood pressure, for a hiatal hernia, and other conditions I had before Parkinson’s. The main thing that happens with Parkinson’s folk, is they become apathetic–about life, or getting out of bed, or even about moving. They become apathetic and a lot of that sinks into depression right away. And I can experience apathy. I’ve never had any depression, which is kind of amazing. They keep asking me, on a scale of one to 10, are you depressed? And they ask it in varying ways, to see if you’re telling the truth, I guess. But I can’t tell anybody about ibogaine. They just go nuts, the medical field.
LN: Have you shared this with any medical people?
Patient D: Well, there are a couple of doctors in New York that are proposing to the government that what the government needs is a full blown, NIH trial, and they’re not able to get any funding. Look at all the body of proof about ibogaine for opioids. There’s really a large body of verifiable truth.
“No effectiveness. It’s not legal in this country. It has no medical uses,” according to the government.
ES: There’s an underground network of providers.
Patient D: Well, I have a list of different symptoms that aren’t normally discussed by doctors. I mean, they don’t tell you about these right away. They do eventually. Okay. These are some of the lesser known functions that are not muscle functions, of the things that happen to Parkinson’s people, that they just can’t tell you (about) because they don’t improve, but I can tell you because I have improved.
Like sleep disorders, depression and anxiety, voice volume, slurred speech. I mean, it was very bothersome when you couldn’t express yourself. Cognitive issues, memory loss, multitasking is something I can’t do anymore. For some reason. I can’t have two things coming at me. I get confused.
So I still have that part of Parkinson’s. I can take care of one thing at a time and follow through and finish it though. They call it orthostatic hypotension which is something I can experience. If you stand up real fast, you get dizzy from that. It’s turned into me actually trying to think of the name now. Lost my sense of balance. Vertigo. I’m learning a new movement technique to counteract it when it happens, because when it happens, I feel like I’m gonna fall over. I haven’t fallen over, but I think I might.
So falling over is the one thing of any illness, where you end up going to the hospital. You hurt yourself. Dystonia, which is muscle contractions of the leg muscles, “restless leg” they call it, but it’s worse than restless leg, like continual.
And the carbidopa levodopa works on me to stop that. So that’s good. The ibogaine doesn’t. They call it bradykinesis— when your face freezes.
LN: I can’t even imagine that. I used to think about it when I saw my mother and I thought it’s like you’re a prisoner inside your own body.
Patient D: Yeah. Kind of like what its like. A prisoner inside your own body.
Apathy and fatigue. Depression and anxiety are one thing, apathy and fatigue are another, entirely separate issue. I can become very apathetic about things unless I get myself jazzed up for something. So sometimes, when I’m in a slump period with Parkinson’s, I don’t feel like doing anything, and I have to push myself through.
So if you lose your sense of edge and you’re wallowing in apathy, it’s a terrible place to be. And I think one of the main benefits that this stuff ibogaine has had on me is mental acuity, so I can still sit and type. I’m getting ready for Dragon Speaking software, for when I can’t type. I don’t drive on the freeway anymore. I can only drive short distances around town because of my eyesight. Parkinson’s affects everything about you. I have terrible seborrhea, like all my skin will just come off — not all of it but just pieces of it dries up and peels off. It’s more embarrassing than it’s painful. Doesn’t bother me. But it bothers the people around me.
And actually, this is the longest I’ve spoken in a long time. I’m tired. It’s something I face every day, but I can deal with it. I’ve got a friend here in town. She can’t even lift the phone to make a phone call. She doesn’t want to. So ibogaine has been a lifesaver for me.
-----
BEFORE YOU CONSIDER TRYING TO MICRODOSE IBOGAINE YOURSELF, PLEASE BE AWARE THAT THERE IS NO MEDICAL RESEARCH TO GUIDE YOU; “PATIENT D’S” STORY IS ALL WE HAVE. IBOGAINE HAS A HALF-LIFE IN THE BODY AS “NORIBOGAINE” AND SO IF YOU MICRODOSE REGULARLY WITHOUT A BREAK YOU MAY EVENTUALLY EXPERIENCE A FULL DOSE’S EFFECTS, WITH ALL ATTENDANT CARDIAC RISKS. IBOGAINE INCREASES THE “QT” INTERVAL OR GAP BETWEEN HEARTBEATS, AND FOR THOSE WITH CERTAIN CARDIAC CONDITIONS, THAT COULD PROVE FATAL. WE DO NOT RECOMMEND THAT ANYONE TAKE IBOGAINE WITHOUT MEDICAL SUPERVISION.

awake.net