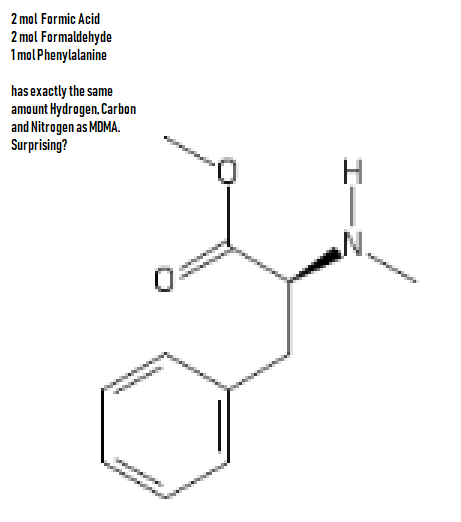
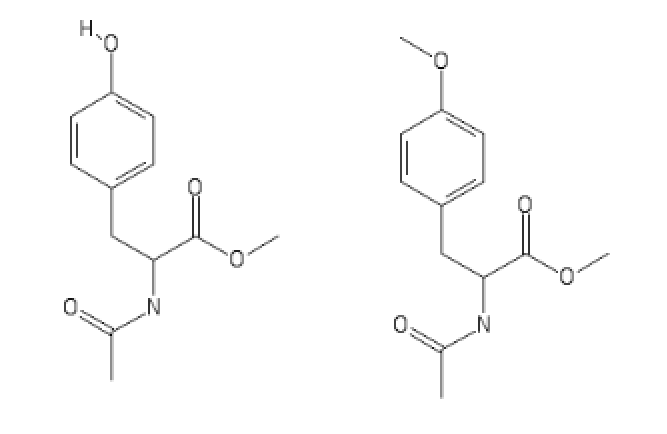
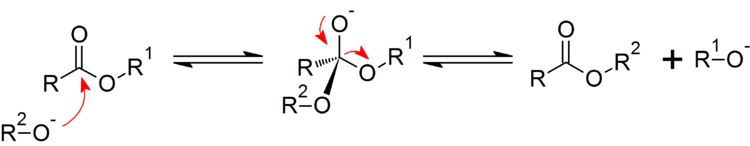Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
FIRST OF ALL: Never snort pure N-Acetyl-Tyrosine; it's the most painful thing I've experienced so far( It does have a kick though).
I'm currently on 5mg Haldol Decanoate/ day and am taking 400mg caffeine and 60mg PseudoEphedrine twice a day to combat depressive feelings of tiredness and laxism that come with it. Will Tyrosine affect my mood and overall stimulation, and how does 500mg of it compare to 60mg Pseudo. I will probably take it in combination with the pseudo in the Morning and skip pseudo at midday.
Also I'll try to make 4-Methoxy Methoxyphenylalannine with it (supposed adrenergic/cholinergic); I'll report back on it.
Do post any experiences or research you've got on the sub.
Older posts of interest:
 www.bluelight.org
www.bluelight.org
 www.bluelight.org
www.bluelight.org
I'm currently on 5mg Haldol Decanoate/ day and am taking 400mg caffeine and 60mg PseudoEphedrine twice a day to combat depressive feelings of tiredness and laxism that come with it. Will Tyrosine affect my mood and overall stimulation, and how does 500mg of it compare to 60mg Pseudo. I will probably take it in combination with the pseudo in the Morning and skip pseudo at midday.
Also I'll try to make 4-Methoxy Methoxyphenylalannine with it (supposed adrenergic/cholinergic); I'll report back on it.
Do post any experiences or research you've got on the sub.
Older posts of interest:
Augmenting NRI's with L-phenylalanine or L-Tyrosine
I find this search engine "google patent" to be pretty good in contrast to "google scholar". https://www.google.com/patents/CA2388377C?cl=en Mentions the treatment of fatigue related to depression being treated with NRI's, in addition to NE precursors, L-phenylalanine or L-Tyrosine. I'vec...
Bupropion(Wellbutrin) & l-tyrosine
I am currently taking Wellbutrin XR daily for ADHD and chronic fatigue, is safe to take l-tyrosine(a dopamine precursor) with Wellbutrin(a dopamine reuptake inhibitor)? Would it give it a boost, be dangerous, or not doing anything at all? Thanks.
Last edited: