Psychedelics as prospective therapeutics for neurodegenerative disorders*
Urszula Kozlowska, Charles Nichols, Kalina Wiatr, Maciej Figiel |
https://doi.org/10.1111/jnc.15509 | 13 September 2021
The studies of psychedelics, especially psychedelic tryptamines like psilocybin, are rapidly gaining interest in neuroscience research. Much of this interest stems from recent clinical studies demonstrating that they have a unique ability to improve the debilitating symptoms of major depressive disorder (MDD) long-term after only a single treatment. Indeed, the Food and Drug Administration (FDA) has recently designated two Phase III clinical trials studying the ability of psilocybin to treat forms of MDD with "Breakthrough Therapy" status. If successful, the use of psychedelics to treat psychiatric diseases like depression would be revolutionary. As more evidence appears in the scientific literature to support their use in psychiatry to treat MDD on and substance use disorders (SUD), recent studies with rodents revealed that their therapeutic effects might extend beyond treating MDD and SUD. For example, psychedelics may have efficacy in the treatment and prevention of brain injury and neurodegenerative diseases such as Alzheimer's Disease. Preclinical work has highlighted psychedelics’ ability to induce neuroplasticity and synaptogenesis, and neural progenitor cell proliferation. Psychedelics may also act as immunomodulators by reducing levels of proinflammatory biomarkers, including IL-1β, IL-6, and tumor necrosis factor-α (TNF-α). Their exact molecular mechanisms, and induction of cellular interactions, especially between neural and glial cells, leading to therapeutic efficacy, remain to be determined. In this review, we discuss recent findings and information on how psychedelics may act therapeutically on cells within the central nervous system (CNS) during brain injuries and neurodegenerative diseases.
------------------------------------------------------------------------------
INTRODUCTION
We are in the midst of a renaissance of research into a class of drugs named psychedelics. This class of drugs was made illegal to use or possess worldwide in the late 1960s, but is now making a comeback as a possible clinical therapy for treating psychiatric conditions such as treatment-resistant depression (TRD), post-traumatic stress disorder (PTSD), and other neuropsychiatric diseases. There is no doubt that psychedelics influence essential functions of the Central Nervous System (CNS). Therefore, they are increasingly recognized and being studied as therapeutic agents for psychiatric disorders. In modern pharmacology, the term "psychedelic" refers to a class of CNS active drugs that primarily produce their effects through serotonin 5-HT2A receptor activation. Classic psychedelics are the natural products: psilocybin, DMT, 5-MeO-DMT, mescaline, and the semi-synthetic ergot derivative lysergic acid diethylamide (LSD). Non-classic psychedelics are newer derivatives of these classic compounds and also include DOx and 2C compounds such as (
R)-DOI and 2C-B. CNS active drugs that can produce similar perceptual alterations such as ketamine, MDMA, and THC are not pharmacologically considered psychedelics because their effects are not mediated primarily through the 5-HT2A receptor. However, recent phase-III-clinical trial data on MDMA-assisted psychotherapy demonstrated therapeutic benefits in patients with severe PTSD.
Research using psychedelics was essentially banned worldwide in the late 1960s and early 1970s, and this class of drug labeled dangerous with no medical value. Fortunately, research in this field has gained interest in recent years, and clinical trials in several areas show promise for these drugs as potential new therapeutics. For instance, so-called "magic mushrooms" are a well-known natural source of the classic psychedelic tryptamine psilocybin. Although known and used for millennia, psilocybin itself was not isolated until 1957 by Albert Hoffman from
Psylocibe mexicana, who first synthesized it in 1958. Psilocybin itself is a prodrug, rapidly converted to the active form, psilocin, in the body. Another classic psychedelic compound, DMT, is found in significant concentrations in several plants such as
Mimosa tenuiflora,
Psychotria viridis, and
Diplopterys cabrerana, among others. It is also produced in the mammalian body but at low levels. DMT was first synthesized in 1931 and isolated in 1942 from
M.
tenuiflora by Oswaldo Gonçalves de Lima. Its psychoactive properties, however, were not confirmed until 1956. The β-carboline and monoamine oxidase inhibitors (MAOi) harmine, tetrahydroharmine, and harmaline in
Banisteropsis caapi are often used to facilitate the oral activity of DMT in the Amazonian brew a
yahuasca, which has also recently been studied for therapeutic benefits.
This review will discuss the current state of the art of how psychedelics influence neural tissue homeostasis and activity. We hypothesize that psychedelics can also be used as therapeutics in the treatment of neurodegenerative diseases and brain injuries. We will mainly focus on neuroimmunology and how data from recent research in the context of neuroinflammation support the hypothesis that psychedelics may have a beneficial outcome in restoring the balance of neural tissue function. In this context, we will also discuss psychedelic-induced neuroplasticity, neurogenesis, and gliogenesis. We propose that psychedelic research in studies of neurodegeneration may be beneficial for future development in this field. We hope that this review will provide information useful to support future psychedelic research in the area of regenerative medicine and the treatment of neurodegenerative disorders and brain injuries.
PSYCHEDELIC INDUCED NEUROPLASTICITY AND NEUROGENESIS
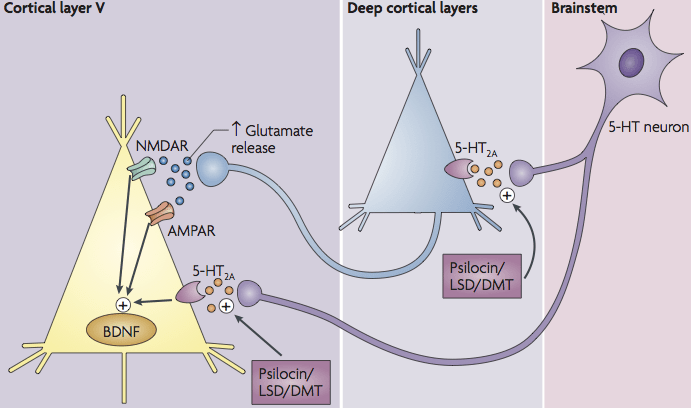
The term "neural plasticity" describes changes in functional neural connectivity. The mechanisms are mostly associated with neural cells, but the process reaches beyond the plasticity of neural synapses. The adaptive changes in the fMRI-measurable macro-scale come from changes in local micro-scales within multicellular interactions, involving neurons, astrocytes, microglia, and oligodendrocytes. These cellular interactions are characterized by complicated homeostatic processes employing both paracrine and direct cell-to-cell communication. Neural plasticity is still poorly understood, but some mechanisms have already been described. Psychedelics may induce a so-called elevated brain entropy state, resulting in an increased ability to learn and "unlearn" certain information. Such action may be therapeutic, and is likely associated with increased neural plasticity mechanisms at the cellular level. Acute changes in the density and complexity of synaptic architecture induced by psychedelics and 5-HT2A receptor activation have been shown by several investigators in both in vitro and in vivo models. These changes involve multiple mechanisms. For example, increases in spine density and morphology can involve direct signaling downstream of 5-HT2A receptor stimulation by psychedelics through serotonylation and activation of Rac1 and kalirin-7, or indirect modulation of synaptic architecture by elevated glutamate levels acting through BDNF/TrkB and mTOR signaling (Figure
1). A feature of psychedelic therapy is the long-lasting effect after only a single treatment. The reason for this is unclear, but likely involves changes in gene expression and/or epigenetic factors underlying the maintenance of neural processes normalized by treatment. There are several known genes involved in synaptic plasticity whose expression is changed in response to psychedelics. Neurogenesis may also be a factor; the administration of DMT induces neural progenitor cells proliferation and adult hippocampal neurogenesis in vivo via activation of Sigma-1 receptors in C57BL/6 mouse. Taken together, neurotrophic signaling and neuroplasticity promoting pathways activated by psychedelics are hypothesized to be key to the mechanism(s) of action for therapeutic effect(s).
A potential key mechanistic component not taken into account for nearly all proposed models is the involvement of microglia for therapeutic effect, as most if not all attention has been focused on neurons in the mechanism of action of psilocybin and other psychedelics. Microglia are tissue-specific, self-renewable CNS macrophage-like cells that are different from other cell types since they appear in the brain and spinal cord during fetal development in the process of primitive hematopoiesis. During their life-long residency inside the CNS environment, microglia assume specific immune cell characteristics and functions. Microglia are very mobile, continually scanning the environment, ready to respond to injury and infections, and take an active part in synaptic rearrangement and neural tissue regeneration. They modulate the deletion of unnecessary connections and the formation of new ones. It is tempting to speculate that psychedelics may stimulate neural plasticity through microglia regulation, especially since many receptors targeted by certain psychedelics like psilocybin and LSD are also present on microglia, including 5-HT2A, 5-HT2B, and 5-HT7 receptors, and the Sigma-1 receptor. Interestingly, in vitro application of DMT and 5-MeO-DMT to monocyte-derived dendritic cells (moDCs) reduce mRNA and protein expression of IL-1β, IL-6, TNF-α, IL-8, and increase expression of regulatory and tolerogenic IL-10.
Another interesting phenomenon is the reciprocity in the dynamics of neuron–microglia interactions. For instance, activation of NMDA receptors on a single neuron's dendrites can stimulate the growth of microglial extensions. Further research may help better understand how neuronal–microglia interactions affect learning and memory, neurodegeneration, and possibly the progression of certain mental illnesses. Microglia may be regulating synaptic pruning or growth by signals from neurons themselves. These regulatory signals may rely on the electrochemical transmission or the complement system, which is also involved in the process of synaptic pruning. During this process, unnecessary synapses are tagged with specific complement proteins that are detected and phagocytized by microglia. Errors in this process during childhood may lead to the development of autism, schizophrenia, or mental retardation, and in adult also result in degenerative diseases. The cellular phenotype and activity of microglia, the involvement of the complement system, and the neuronal signals relevant to synaptic plasticity upon psychedelics stimulation are probably critical aspects of the psychedelic therapeutic mechanism.
Our unpublished data suggest that psilocin increases the protein expression of triggering receptor expressed on myeloid cells 2 (TREM2) on microglia while reducing p65, TLR4, and CD80 proinflammatory markers. TREM2 is involved in the regulation of several microglial functions, including phagocytosis and synaptic refinement. Microglia deficient in TREM2 expression results in synaptic pruning defects, increased excitatory neurotransmission, and reduced long-range functional connectivity. The down-regulation of TREM2 was also observed in brain samples of patients suffering refractory epilepsy. According to our pilot data, psychedelics may prevent neuronal damage in microglia-neuron co-culture, however, it is presently unknown if the protective mechanisms of psychedelics are mediated by microglial TREM2.
PATHOLOGICAL MECHANISMS IN NEURODEGENERATION—A POTENTIAL TARGET FOR PSYCHEDELICS
Because the brain is very fragile and hardly an accessible organ, only limited therapeutic approaches can be proposed for the treatment of brain-specific neurodegeneration. These can be pharmacological, stem cell, or gene therapy approaches. Unfortunately, results to date with these approaches have not been very successful. Interestingly, one recently proposed solution is the application of traditional psychiatric drugs because they have been shown to prevent neural loss and stimulate neurogenesis, Neuroprotection and induction of neurogenesis may be a fruitful avenue to treat MDD as the histopathology of depressed individuals sometimes show signs of subtle neurodegeneration. For example, post-mortem brain studies have revealed neural loss and atrophy in the prefrontal cortex (PFC) and the hippocampus. Neural protective mechanisms (e.g., neuroprotection, neurogenesis, neuroplasticity) have been shown to be induced by psychedelics, which are effective in the treatment of MDD. Here, we discuss pathologies that occur in neurodegenerative disorders that may potentially be targeted by psychedelics for therapeutic effect. We speculate that their application at early disease stages may result in the delay of pathological symptoms.
Oxidative stress
Oxidative cell damage is often a reported in brain-specific neurodegeneration. This damage usually occurs because of an imbalance between free radicals, reactive oxygen species (ROS), and reactive nitrogen species (RNS), and the presence of antioxidants and antioxidative proteins, such as superoxide dismutases (SOD), hioredoxin peroxidases (TRXPs), glutathione peroxidases (GPXs). In a typical situation, if reactive species are held in balance, they play an essential role in regulating essential cellular processes including phagocytosis, apoptosis, and cellular signaling. However, when cells are unable to neutralize excesses of reactive molecules, these molecules may induce damage to mitochondria, cellular and nucleolar membrane, and DNA, and over time result in organ and/or tissue degeneration. Elevated oxidative stress and disruption in redox balance are observed in many psychiatric conditions such as MDD, schizophernia, bipolar disorder, anxiety disorder. Importantly, disruption of redox homeostasis occurs in the pathology of ALS, PD, AD, and DNA repeat expansion disorders such as HD and SCAs.
In the psychedelic brew ayahuasca, two components, harmine and harmaline, are monoamine oxidase inhibitors with antioxidant properties and have the capability to induce gliogenesis and neural progenitor cell migration. Another anti-oxidative effect of psychedelics such as psilocybin and/or DMT may come as a result of 5-HT1A receptor activation. The 5-HT1A receptor agonist 8-OH-DPAT induces expression of the anti-oxidative factor metallothionein-1/-2 (MT-1/-2) and Nfr2. In retina pigment epithelial cell line (ARPE-19), 8-OH-DPAT reduces damage caused by paraquat, an oxidative herbal agent, through elevation of
MT1,
heme oxygenase-1
(HO1),
NAD(P)H:
quinone acceptor oxidoreductase 1 (NqO1),
superoxide dismutase 1 and 2 (
SOD1,
SOD2), and
catalase (
Cat) mRNA expression. 8-OH-DPAT also reduces oxidative stress damage in retinal pigment epithelium/choroid in
Sod2 knockout mice.
Endoplasmatic reticulum stress (ERS)
Some psychedelics (e.g., DMT) target the Sigma-1 receptor, which is reported to protect cells from various insults. ER stress induces up-regulation of Sigma-1 expression and modulates the action of PERK, IRE1α, and ATF6 proteins in mitochondria-associated membrane (MAM). Stimulation of Sigma-1 may prevent ERS-mediated cellular apoptosis by regulation of ATF4, ATF6/ C/EBP homologous protein (CHOP), and the balance between Bax and Bcl-2 in granulosa cells. Because ERS damage is reported in MDD and several neurodegenerative disorders, targeting Sigma-1 receptors with psychedelics is proposed as a novel therapeutic strategy.
Blood–brain barrier disruption
The vasculature system in the brain is equipped with a special feature called the blood–brain barrier (BBB). The BBB is composed of a tight layer of astrocytes, is selectively permeable, and separates the intracerebral circulatory system from the peripheral blood to protect the brain against chemical and biological insults. The BBB also contains other cells types, such as microglia cells, perivascular macrophages, and pericytes. The whole structure is embedded in the basal membrane, with extracellular matrix secreted by endothelial cells and pericytes. The endothelial cells inside the blood capillaries form tight junctions (TJ), multi-protein complexes composed of occludins, claudins, and tight junction proteins ZO-1, -2, -3. Breakdown of this system is associated with brain-specific damage and neurodegeneration, and may be the cause of serious illness. Breakdown can originate from prolonged exposure to oxidative stress and/or immune cell activity. For example, microglia inflammatory cytokines acting via IL-1β on Sonic Hedgehog (SHH) can down-regulate tight-junction proteins in astrocytes, resulting in BBB leakage. Moreover, suppressing SHH in astrocytes leads to increased secretion of proinflammatory chemotactic proteins and immune cell activation. Microglia also secrete IL-1β via inflammasome-dependent mechanisms in response to proinflammatory cytokines, DAMPS, β-amyloid, or other toxic protein aggregates. In a C57BL/6 healthy male mouse model, chronic social stress causes BBB disruption via claudin-5 down-regulation, which leads to the infiltration of proinflammatory factors and depression-like behaviors. Disruption of the BBB is also observed in a genetic mouse model of schizophrenia, and may be involved in bipolar disorder pathology. BBB disruption is also observed in multiple neurodegenerative disorders including ALS, AD, and SCA3.
Conceptually, inflammation-based BBB leakage could be prevented to some degree by the presence of psychedelics. The drugs N,N-DMT and 5-MeO-DMT, applied into LPS and polyI:C – activated dendritic cells in vitro, result in down-regulation of expression of IL-1β, IL-6, IL-8, TNF-α, and up-regulation of IL-10 as measured by mRNA and protein expression through stimulation of Sigma-1 receptors. This observation has also been confirmed in vivo in a Wistar rat model of stroke, where N,N-DMT administration significantly decreased IL-1β, IL-6, and TNF-α, but increased IL-10, measured by mRNA and protein expression. Furthermore, N,N-DMT-treated rats demonstrate improved motor skills post-stroke. Although not validated yet in brain tissues, several psychedelics, including DOI, LSD, and psilocybin, have been shown to have potent anti-inflammatory effects of suppressing many of these same proinflammatory biomarkers in peripheral tissues, and they may represent effective therapies for inflammation-related neuropathologies.
Oligodendrocyte pathology
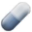
Oligodendrocytes protect and support neurons and their axons by providing myelin that improves electric signal transmission. Unfortunately, their active role in immune response and neural regeneration has long been overlooked. Microglia and oligodendrocytes actively work to regulate each other's functions. Moreover, oligodendrocytes’ pathology occurs in many neurodegenerative diseases, including Alzheimer's and Parkinson's Disease, ALS, Multiple Sclerosis, Spinal Cord Injury, but also non-degenerative psychiatric conditions including MDD, schizophrenia, and Alcohol Use Disorder. For example, in MDD, abnormalities in oligodendrocyte density are observed in the PFC and amygdala. Interestingly, oligodendrocytes are extremely vulnerable to oxidative stress and prolonged exposure to proinflammatory factors secreted by microglia. The previously discussed psychedelic-mediated reduction in cytokine secretions may play a protective role in myelin and oligodendrocyte cell survival. Certain psychedelics can target Sigma-1 receptors, which are essential in stimulating OPC differentials. Together, these findings indicate that more attention should be paid to the influence of psychedelics on oligodendrocyte biology.
PSYCHEDELICS AS IMMUNOMODULATORS
The study of psychedelics at target receptors and the activation of effector pathways have brought new, but still limited, insights into their immunomodulatory potential (see Table
1). Classic psychedelics like LSD, DMT, 5-Meo-DMT, and psilocin have the potential to interact with several 5-HT receptor subtypes, Sigma-1R, and TAAR, which are present in CNS and other tissues, including cells of innate and adaptive immunity like macrophages, monocytes, dendritic cells, and T cells. These receptors are mediators of immunological response, and serotonin is considered a critical factor in immune homeostasis. Therefore, psychedelics can regulate both adaptive and innate immune responses. A review of putative molecular mechanisms in which psychedelics may act as immunomodulators was published by Szabo, emphasizing cross-talk between pattern recognition receptors (PRR), such as Toll-Like Receptor 4 (TLR4), 5-HTRs, and Sigma-1R, and regulation of inflammatory response via NFκB/IRF signal transduction pathways. Although these modulations result in changes, Sigma-1, and TAAR seem to play a crucial role in immune response, and all three of them can be stimulated by psychedelics.
| Psychedelic ligands | Receptor | Effect | Literature |
|---|
| (R)- DOI | 5-HT2A | In smooth muscle cells in vitro:
Prevention of Nf-κβ nuclear translocation and inhibition of nitric-oxide synthase activity
In aortic arch and small intestine in vivo:
Down-regulation of TNF-α-mediated Cx3CL1, Icam-1, Vcam-1, MCP-1, IL-6, IL-1β
In OVA-treated asthma-model lung in vivo:
Suppression of Th2-related genes: Mcp-1, Il-13, Il-5, and Gm-csf,
Inhibition of neutrophil infiltration,
Inhibition of mucus hyperproduction | Yu et al. (2008)
Nau et al. (2013)
Nau et al. (2015 |
DMT,
5-MeO-DMT | Sigma-1 | Down-regulation of: IL-1 β, IL-6, TNF-α, IL-8
Up-regulation of: IL-10
(gene and protein expression)
Decrease of Th1 and Th17 activation after E. coli or H1N1 co-culture | Szabo et al. (2014) |
| 5-MeO-DMT | 5-HT2A (?)
5-HT2c (?) | In human cerebral organoid in vitro model:
Down-regulation of Nf-κβ pathways (involved in the immune response)
Down-regulation of Nuclear Factor of Activated T cells (NFAT) (T-cell activation, stem cell differentiation)
Modulation of Gaq-Rho-ROCK pathway (cytoskeletal rearrangement, phagocytosis) | Dakic et al. (2017) |
| DOI, DMT, Psilocin | 5-HT2B | (Reported with BW723C86 5-HT2B ligand)
In CD1+ moDC down-regulation of: TNF-α, IL-6, IL-8/CXCL8/ IP-10/CXCL10 after TLR2, and TLR6/7 activation,
In TLR3-activated CD1+ moDC: down-regulation of CD80, CD83, CD86 (anti-inflammatory, tolerogenic)
Prevention of Th1, Th17 lymphocyte polarization | Szabo et al. (2018) |
| DMT | Sigma-1 | In in vivo model of stroke:
Down-regulation of: IL-1β, IL-6, IL-8, TNF-α, NOS, APAF-1 (proinflammatory, proapoptotic)
Up-regulation of BDNF and IL-10 (tolerogenic, neurogenic) | Nardai et al. (2020) |
| (Hypothetically) DOI, DMT, Psilocin, LSD, mescaline | TAAR1, TAAR2 | (Not tested yet with psychedelics)
Lymphocyte migration, increase in IL-4 secretion, Th1/Th2/Th3 phenotype modulation, mediation of IgE-secretion | Babusyte et al. (2013) |
5.1 5-HT receptors
Serotonin is one of the most critical factors during fetal brain development and neurogenesis, and is responsible for the formation of axons and dendrites, and adult axonal regeneration. Serotonin receptors are present on most, if not all, types of cells in the CNS. In neurons, for example, their activation can influence cellular membrane polarization states through multiple mechanisms. Serotonin also plays significant roles aside from being a neurotransmitter. There are several receptor subtypes expressed in mammalian peripheral tissues and cells outside the CNS, including adaptive and innate immune cells. Serotonin itself has an endocrine effect on the regulation of whole-body homeostasis, such as heart rate, intestinal motility, and last but not least: the immune response.
Although 5-HTRs are primarily described as activators of proinflammatory pathways, they surprisingly have anti-inflammatory properties when activated by certain, but not all, psychedelics. The selective 5-HT2 receptor agonist (
R)-DOI, reduces mRNA expression of proinflammatory adhesion molecules ICAM-1 and VCAM-1 as well as mRNA levels for proinflammatory cytokines MCP1, IL-1β, and IL-6 in various tissues like intestine and aorta, and circulating levels of IL-6 in TNF-α treated mice. Several of these findings were confirmed in a high fat-fed ApoE−/− knockout moue model of cardiovascular and metabolic disease. An increase in levels of VCAM-1, IL-6, and MCP-1 mRNA expression was observed in animals fed a high-fat and -cholesterol "Western diet" compared to control mice fed regular food, and this increase was prevented in mice fed the Western diet and treated with (
R)-DOI. The precise mechanism underlying why 5-HT2A receptors, which are widely described as inflammation inducers, induce anti-inflammatory processes after activated by (
R)-DOI and some other psychedelics is not known. The hypothesis proposed by Flanagan and Nichols involves the concept of functional selectivity, in which different ligands induce slightly different conformations of the receptor to recruit different sets of effector pathways. Psychedelics are hypothesized to recruit and activate anti-inflammatory effector signaling pathways, whereas serotonin itself recruits proinflammatory pathways. In rodent models of allergic asthma, nasal administration of (
R)-DOI at a very low dose (EC50: ~0.005 mg/kg) completely prevents symptoms, including airways hyperresponsiveness, pulmonary inflammation, and mucus overproduction in response to allergen. Further examination of the lung tissue revealed prevention of eosinophilia and a reduction in Th2 cell recruitment. Interestingly, the behavioral potency of different psychedelics does not correlate with anti-asthma efficacy. Significantly, therapeutic drug levels in these models are orders of magnitude lower than the levels necessary to induce measurable behavioral responses. These findings suggest that subperceptual levels of some psychedelics may be an exciting alternative to currently available steroid drugs in the treatment of asthma and other inflammatory-related disease.
Given the high level of expression of 5-HT2A receptors in the brain on multiple cell types, it may be that psychedelics have similar anti-inflammatory properties against neuroinflammation. In vitro application of 5-MeO-DMT in human cerebellar organoids results in down-regulation of NF-κβ and nuclear factor of activated T cells (NFAT) pathways, as well as modulation of the Gαq-RhoA-ROCK pathway involved in cytoskeleton rearrangement and phagocytosis.
Classical psychedelics have mid affinity for and efficacy at 5-HT2B receptors. Interestingly, the activation of this receptor type with the agonist (BW723C86) regulates immune responses in CD1+ monocyte-derived dendritic cells (moDC). The application of BW723C86 resulted in down-regulation of CD80, CD83, and CD86 proinflammatory molecules on CD1+ moDC. Furthermore, stimulation of 5-HT2B down-regulates TLR2, TLR3, and TLR7/8-mediated proinflammatory cytokine protein expression. It also prevents moDC-mediated activation of T cells toward inflammatory Th1 and Th-17 phenotypes. Furthermore, certain immune stimulators such as the molecule polyI:C, which is a TLR3 agonist, up-regulate the expression 5-HT2B receptor protein. Together, these observations suggest that 5-HT2B agonism may participate in some aspects of their anti-inflammatory mechanism. However, in the allergic asthma model,
(R)-DOI was not effective in reducing pulmonary inflammation in the 5-HT2A receptor knockout mouse indicating that for at least asthma and pulmonary inflammation 5-HT2A receptor activity is necessary and sufficient for therapeutic effect.
5.2 Sigma-1 receptor
The sigma-1R is a transmembrane protein located in mitochondria and the endoplasmatic reticulum (ER). It is abundantly present within the CNS in neurons, astrocytes, oligodendrocytes, and microglia, where it mediates a neuroprotective effect. Sigma-1R activity promotes neural function and survival via modulation of Ca2+ homeostasis, mitigation of oxidative stress, regulation of gliosis, neuroplasticity, and glutamate activity.
Stimulation of Sigma-1R in oligodendrocyte progenitor cells (OPC) results in oligodendrocyte differentiation, and stimulation in astrocytes improves the BDNF secretion. This suggests that targeting Sigma-1R may be a promising therapeutic strategy for psychiatric and neurodegenerative conditions. Interestingly, Sigma-1R activity is involved in the transition between M1-like proinflammatory and M2-like proregenerative and tolerogenic microglia phenotypes. These mechanisms are not very well understood. Moreover, as microglia are cells of complicated biology and are somewhat difficult to study, the concept of M1/M2 polarization may be too simplistic to address many aspects of microglia function. It has been recently proposed that microglia displaying a proinflammatory phenotype are crucial for their role in neural tissue reorganization and regeneration.
DMT is an agonist of Sigma-1Rs, and found to be produced by specific tissues in the brain. Szabo et al. observed that the application of DMT into human iPSC-derived cortical neuron cultures in vitro resulted in better survival under hypoxia conditions, but that the protective effect vanished after Sigma-1R gene knockdown with siRNA. This suggests that DMT can protect cells from hypoxia-induced apoptosis via Sigma-1 receptor stimulation. This observation was later confirmed in an in vivo rat model of stroke, where continuous administration of DMT reduced the size and number of lesions, and decreased levels of IL-1β while up-regulating IL-10, and BDNF protein and mRNA levels. Similar results were found with another Sigma-1 agonist (PRE-084) after embolic stroke to significantly reduce the size of lesions, improve neuronal deficits, and reduce concentrations of some proinflammatory cytokines while elevating levels of some anti-inflammatory cytokines like IL-10. Interestingly, application of the Sigma-1R selective antagonist (MR309), had similar neuroprotective effects. It is tempting to speculate that elevated anti-inflammatory cytokine levels after DMT administration in these stroke models may be caused by Sigma-1R mediated changes in microglia phenotypes. For example, in a study by Moritz et al., stimulation of Sigma-1R "switched off" activated microglia and made them migrate away from the location of damaged tissue. Moreover, in the LPS-treated microglial BV2 cell line, application of Sigma-1R agonist SKF83959 results in the prevention of M1-like phenotype switching by microglia, and a decrease in TNF-α, IL-1β, and inducible NOS levels. A similar effect was reported in a model of traumatic brain injury (TBI), and Parkinson's Disease.
TAAR
Trace amine-associated receptors (TAARs) are G-protein-coupled receptors abundantly present in the CNS. In most vertebrates, they exist in nine isoforms. Only TAAR1 has been studied in-depth, however. This receptor is relatively non-selective and has an affinity for endogenous trace amines as well as the classical neurotransmitters serotonin and dopamine, and multiple psychoactive drugs, including amphetamines, ergoline derivatives, psilocin, DMT, and mescaline. TAAR1 is a modulator of neurotransmission induced by canonical dopamine, serotonin, and glutamine receptors, and its aberrations and rare variants may contribute to the etiology of schizophrenia. TAAR1 is also expressed in non-CNS tissues such as the thyroid, stomach, pancreas, and intestine, where it may regulate body functions in an endocrine manner.
Although abnormalities in TAARs expression or function may be related to the development of schizophrenia, data suggest involvement in additional neuropsychiatric conditions. For example, stimulation of TAAR1 in an experimental model of Parkinson's Disease results in L-DOPA-related dyskinesias, and TAAR1 knockout mice are reported to be more vulnerable to various substance addiction. Targeting TAAR1 has also been suggested as a possible therapeutic target for the treatment of bipolar depression, fibromyalgia syndrome, and diabetes.
TAAR are found in immune cells and can elicit immunomodulatory effects; however, our knowledge about TAARs and immune responses is limited. TAAR1 is expressed in polymorphonuclear leukocytes (PMN), T cells, and B cells, whereas TAAR2 is also abundant on NK cells and monocytes. In T cells, stimulation of TAAR1 and TAAR2 receptors induce IL-4 production and modulation of Th1, Th2, and Th3 markers, whereas silencing of these receptors reduces IgE secretion in B cells after induction with trace amines. TAAR1 and TAAR2 are also reported to be involved in PMN chemotactic migration. DMT, (
R)-DOI, d-LSD, and 5-MeO-DMT are TAAR1 agonists, and it is, therefore, possible that psychedelics may regulate immune cells to respond and regulate neural tissue homeostasis via TAAR1 activation.
RESEARCH PERSPECTIVES FOR PSYCHEDELICS IN PREVENTING NEURODEGENERATION
In this review, we have highlighted the beneficial outcomes of psychedelic treatment for MDD. Our primary focus was on processes in neural tissue microenvironments which can be affected by psychedelics. These include the induction of neurogenesis and neuroplasticity and reduction of inflammation and oxidative stress. These characteristics of psychedelics may play crucial roles in restoring long-term healthy homeostasis in depressed patients. We also emphasized potential areas of therapeutic actions in brain-specific neurodegeneration in which psychedelics may be beneficial. These include oxidative stress, inflammation, BBB disruption, and loss of oligodendrocytes and myelin.
In Europe, around 7 million people suffer from dementia-related disorders, and the aging of society is expected to double this number by 2040. Around 15 mln people worldwide and 1,12 mln in Europe experience a stroke every year, with 5 mln of those being fatal incidents (650 000 in Europe), and another 5 mln of patients suffer post-stroke severe disability. Each year 10 mln new dementia cases are being diagnosed globally, and 60–70% of them are Alzheimer's Disease. In 2015 ALS was diagnosed in 222 801 people worldwide, and that number is predicted to grow by 69%, reaching 376 674 new ALS cases annually by 2040. Therefore, neurodegenerative diseases are a serious and growing burden for modern societies. Moreover, the development of effective therapeutics lags behind other fields such as cardiovascular diseases and cancer. Therefore it is a top priority to search for novel candidates for therapeutic approaches to revert these dire statistics.
Psychedelics represent such a novel approach. Certain psychedelics, with demonstrated therapeutic efficacy for psychiatric disorders in clinical trials, have been used safely for centuries by indigenous populations. The beneficial therapeutic dosages of these substances have been shown to be well-tolerated, and they present a favorable safety profile in treating a variety of disorders. Clinical trials are investigating therapeutic efficacy for anorexia nervosa, the early stage of Alzheimer's Disease, and traumatic brain injury, among other CNS disorders.
Psychedelics (e.g., DOI, DMT, LSD) promote structural plasticity via BDNF signaling and are thus proposed as potential therapeutics for MDD and related disorders. Neuropsychiatric disorders are well known to be associated with atrophy of neurons and abnormal neuronal circuits. Among neurodegenerative disorders, commonalities in pathological characteristics may be seen in polyQ disorders such as spinocerebellar ataxia type 3 (SCA3).
Similar to many other neurodegenerative disorders, the down-regulation of BDNF is observed in SCA3 cells and in dentate neurons of SCA3 patients. Moreover, several essential proteins belonging to the BDNF signaling pathway are also down-regulated in mouse models. For example, Rac1, acting downstream of BDNF and TrkB, is down-regulated in the cerebral cortex and cerebellum of young SCA3 mice. Together with another down-regulated protein, RhoA, Rac1 mediates proplasticity properties evoked by BDNF by facilitating sLTP (structural long-term potentiation) and regulating actin cytoskeleton in dendritic spines. Furthermore, MAPK1 (a.k.a. Erk) and MAP2K1 are also down-regulated in the young SCA3 mice, and Erk signaling is down-regulated in MDD. Importantly, BDNF is an immediate upstream regulator of the MAPK (mitogen-activated protein kinase) cascade. Activation of MAPK (ERK) signaling by neurotrophins is involved in long-term synaptic plasticity and the structural remodeling of the spines in the excitatory synapses.
Another protein necessary for BDNF signal transduction to the nucleus is Pea15, which is also down-regulated in SCA3 mice. Silencing of Pea15 results in inhibition of BDNF retrograde signaling. Pea15 acts as a scaffolding protein for PLD1, RSK2, and ERK1/2, and the formation of this complex is triggered by BDNF in cortical neurons. Therefore, the potential use of psychedelics could affect the levels of several proteins, which are down-regulated in the SCA3 model probably by an increase in BDNF which may promote synaptic plasticity. The role of BDNF signaling in the survival of neurons has been well documented in other neurodegenerative disorders, such as Huntington's, Parkinson's, and Alzheimer's disease.
Psychedelics have also been shown to reduce oxidative stress, which is a significant issue in neurodegenerative disorders. Several oxidative stress biomarkers are elevated in models of neurodegenerative disorders. Other anti-oxidative proteins, which play an essential role in reducing oxidative stress by breaking down ROS, are down-regulated in the SCA3 model. Moreover, Txn down-regulation in SCA3 mice might increase the vulnerability of neurons to ROS. Therefore, psychedelics’ anti-oxidative properties could be a beneficial component of a therapeutic strategy for SCA3 and other neurodegenerative disorders.
The strategy of serotonergic signaling modulation by psychedelics in SCA3 is also strongly supported by studies showing a therapeutic effect for SCA3 through 5-HT1AR activity, which is activated by several psychedelics including psilocybin and LSD. Targeting of the 5-HT1A serotonin receptor orthologue SER-4 in
C.
elegans ameliorates motor dysfunction and reduced mutant ATXN3 aggregation. Furthermore, treatment with partial agonists of 5-HT1A receptors has been demonstrated to reduce ataxia, pain, insomnia, and depressive symptoms in patients with SCA3 and other forms of SCA. The SSRI citalopram has beneficial therapeutic effects in animal models of SCA3 and preclinical trials. Thus, activation of serotonergic signaling in SCA3 patients with psychedelic agents is a promising therapeutic strategy.
Non-hallucinogenic psychedelics approach
The “hallucinogenic” effects of psychedelics have been proposed to be directly associated with their therapeutic potential in psychedelic-assisted psychotherapeutical approach as the subjective peak intensity has a high correlation with therapeutic efficacy. However, in patients suffering brain-specific neurodegeneration, psychotropic effects of psychedelics may be a serious limitation, especially if because of the disease physiology, the medicine would have to be administrated more often and in higher doses. Furthermore, correlation is not causation, and subjective peak experiences may merely indicate that sufficient drug has been administered to produce therapeutic efficacy at cellular and molecular targets and circuits. Although ibogaine is not classified as a psychedelic, it is a type of hallucinogen that may have therapeutic efficacy to treat substance use disorder. Recently, analogs of ibogaine have been reported by two different investigators to attenuate behaviors associated with substance abuse in rodent models, in a similar way to ibogaine. One is peer reviewed, and the other awaiting peer review. Because of the low toxicity and lack of hallucinogenic properties of these new molecules, they represent potential non-hallucinogenic derivatives of hallucinogenic parent molecules with therapeutic effect. With regard to psychedelics, their demonstrated potency in multiple animal models of disease suggests it may not be necessary to eliminate hallucinogenic behaviors from effective molecules because the dose is so low that effects on behaviors would not be seen at relevant therapeutic levels. Regardless, work by Flanagan et al. suggest that it may be possible to engineer hallucinogenic effects away from therapeutic effects to develop non-hallucinogenic 5-HT2A receptor agonists with anti-inflammatory potential. Development of a 5-HT2A receptor agonist therapeutic devoid of hallucinogenic effects would conceivably allow higher levels to be used, especially for those with weaker potencies, which may allow for greater efficacy in certain circumstances.
Prospect implications for regenerative medicine
Besides the prevention and regulation of pathology in neurodegenerative disease, the immunomodulatory properties of psychedelics may also be relevant to regenerative medicine. Neural Stem/Progenitor Cell (NSC/NPC) transplantation is a recently developed and promising therapeutic tool. However, the limitation of such a strategy is poor graft survival because of immune response. Our recent study revealed that DMT and psilocin down-regulate CD80 co-stimulatory molecule expression on the surface of microglial cells, with and without LPS stimulation. The co-stimulatory signal is crucial for recruiting adaptive immune cells; therefore, blockade of co-stimulatory molecules an attractive immunosuppressive strategy. The unique properties of psychedelics in suppressing inflammatory responses, and promoting neural survival and plasticity, could be a strong rationale for the hypothesis that psychedelics might support grafted cells and facilitate their survival for therapeutic benefits.
Therapeutic perspectives for microdosing
One dosing method of psychedelics is the use of so called “microdoses”—very low concentrations of various psychedelics that do not reach the threshold of perceivable behavioral effects. This is usually 10% of active recreational doses taken up to three times per week. This regimen is popular in underground settings without medical guidance. Microdosing is believed to improve the creative thinking, cognitive function, and overall psychological well-being, and is described mostly in the context of self-application by healthy enthusiasts. There have been few rigorously controlled studies of microdosing, and the therapeutic effects of psychedelic microdoses for the treatment of psychiatric disorders are questionable. According to a self-blinding study involving 191 healthy volunteers, in which the mood changes were measured using various questionaries, the authors concluded that anecdotal psychological improvements are more likely associated with the placebo effect rather than drug effect. Furthermore, Family et al. reported that repeated administration of LSD (5–20 µg) in healthy individuals in a blinded placebo-controlled clinical trial produced no significant changes in several cognitive outcome measures. However, a recent study using fMRI showed that 13 µg of LSD changes connectivity inside the limbic circuits 90 min after drug administration compared to the placebo control, which was associated with positive mood changes as measured with a Positive and Negative Affect Schedule (PANAS). Each of these reports has been in healthy individuals, and there have been no rigorous and controlled studies to date on microdosing in patients with diagnosed depressive disorder. The application of psychedelic microdosing in the context of the treatment of brain-specific neurodegenerative disorders has not been yet directly investigated, however, researches speculate that it may influence the hippocampal neurogenesis. Importantly, a Phase I feasibility and safety study on repeated low-dose LSD administration has been conducted in an elderly healthy population in preparations for later phase clinical trials to treat Alzheimer's Disease.
CONCLUSION
Psychedelics stimulate neuro- and gliogenesis, reduce inflammation, and ameliorate oxidative stress. Therefore, they are promising candidates for future therapeutics for psychiatric, neurodegenerative, and movement disorders. Importantly, psychedelics hold the promise of being disease-modifying therapeutics, and not simply just providing symptomatic relief. Current clinical trials have demonstrated both safety and efficacy for their therapeutic use in controlled clinical settings, and psilocybin, has even been designated with “Breakthrough Therapy” status by the FDA in the United States for two different Phase III clinical trials. Often only just one or two therapeutic administrations produce profound and persistent effects. Preclinical research has shown promise in several disease models for both psychiatric and non-psychiatric diseases. Therefore, the use of psychedelics as therapeutics is very promising and should be further developed, paying special attention in the future to prospect applications in neurodegenerative diseases.
*From the article (including references) here :

 www.bluelight.org
www.bluelight.org