haribo1
Ex-Bluelighter
- Joined
- Nov 29, 2006
- Messages
- 4,826
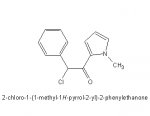
I've been interested in this compound for years. After the Second World War, the Japanese public, desperate to rebuild their shattered economy & infrastructure went on one almighty binge to help them work. Amphetamines were cheaper than food and allowed the beaverlike Japanese to work even harder. The drug they went for wasn't (meth)amphetamine, ritalin or anything like. It was this:

OK, it looks sort of PEA derived but its action is quite interesting. Not only does it stop the reuptake of dopamine & norepinephrine but it also binds with the opiate receptors. Now, it doesn't follow the morphine rule (1-aromatic system 2-Quaternary carbon 3-two carbon chain, 4 tertiary amine) but then again, their are a few other agents that don't. Tilidine being a good example.
It is reported to have both stimulant & 'opiate like' effects everywhere I read. It produces opiate tolerance & dependance that naloxone precipitates withdrawal syndrome. It is usually described as a 'partial agonist'.
So, stimulant AND opiate. Sounds like a speedball with one chemical, doesn't it. I know the N,N dimethyl has replaced with diethyl & ortho morphilino methyl homalogues but no details.
I'm thinking that this could be a very interesting compound, if skillfully manipulated.
I'm wondering if anyone has researched or has access to research papers on this and related structures.

OK, it looks sort of PEA derived but its action is quite interesting. Not only does it stop the reuptake of dopamine & norepinephrine but it also binds with the opiate receptors. Now, it doesn't follow the morphine rule (1-aromatic system 2-Quaternary carbon 3-two carbon chain, 4 tertiary amine) but then again, their are a few other agents that don't. Tilidine being a good example.
It is reported to have both stimulant & 'opiate like' effects everywhere I read. It produces opiate tolerance & dependance that naloxone precipitates withdrawal syndrome. It is usually described as a 'partial agonist'.
So, stimulant AND opiate. Sounds like a speedball with one chemical, doesn't it. I know the N,N dimethyl has replaced with diethyl & ortho morphilino methyl homalogues but no details.
I'm thinking that this could be a very interesting compound, if skillfully manipulated.
I'm wondering if anyone has researched or has access to research papers on this and related structures.