mr peabody
Bluelight Crew
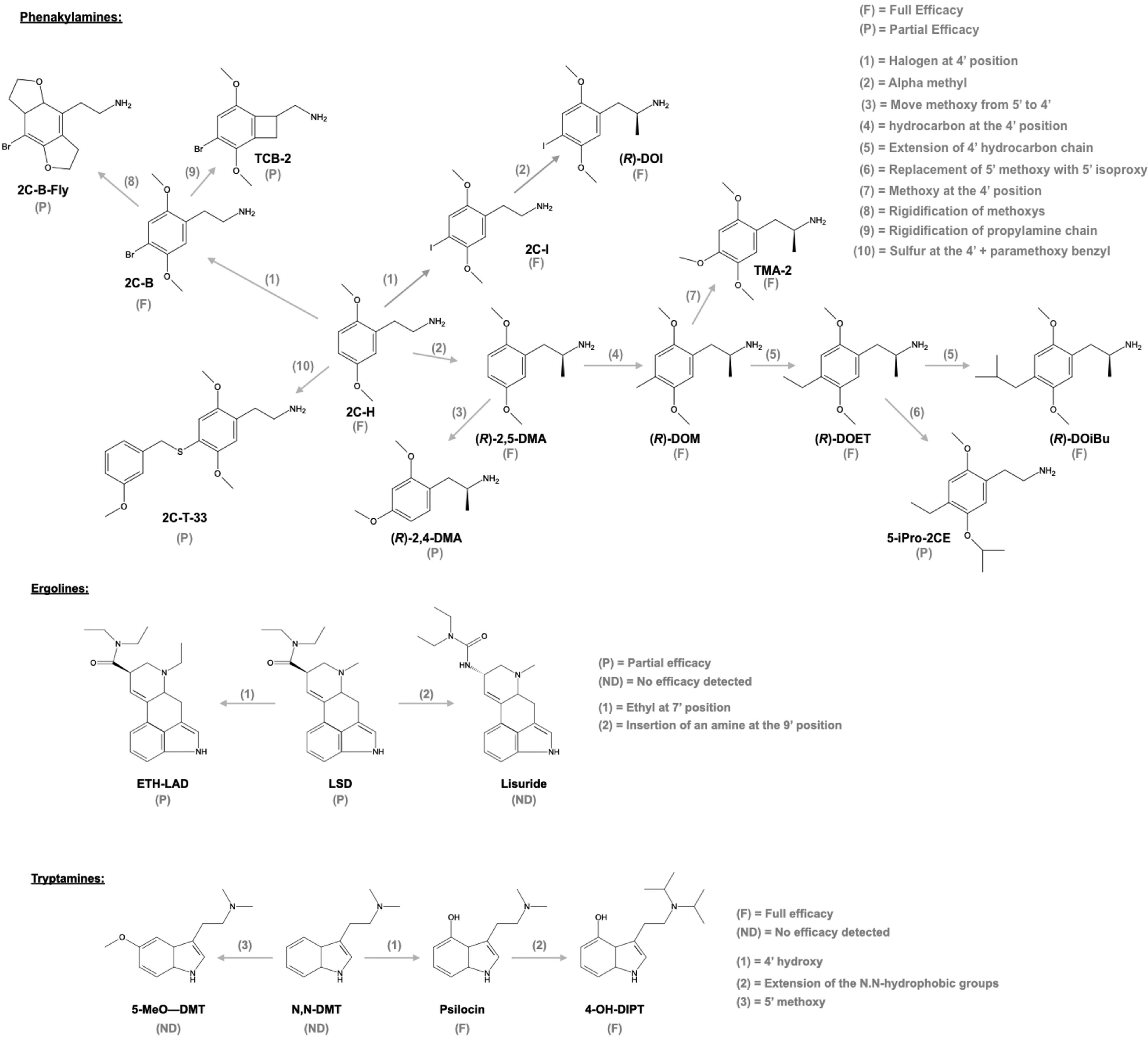
Psychedelics as anti-inflammatory agents*
by Thomas Flanagan & Charles Nichols | 13 Aug 2018
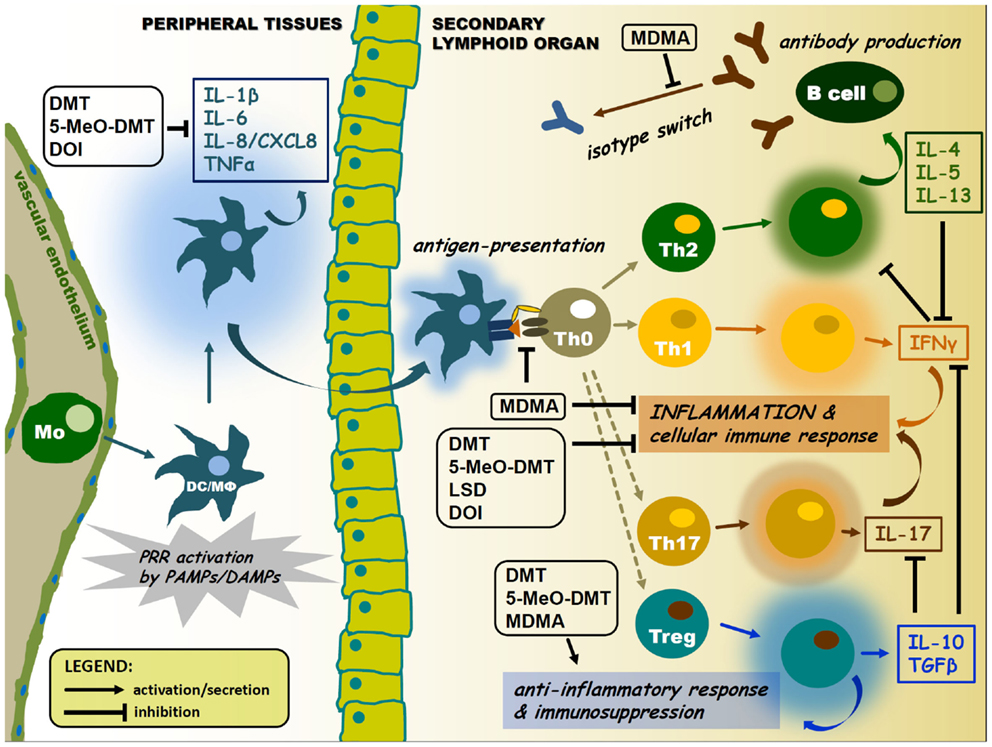
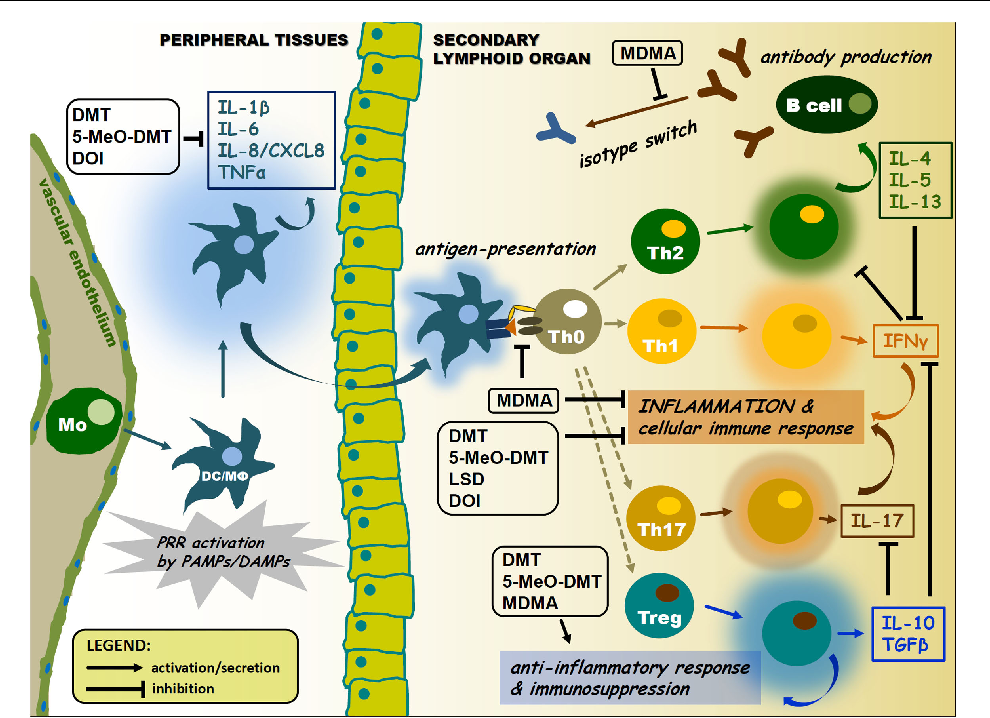
Serotonin receptor agonists have recently emerged as promising new treatment options for a variety of disorders. The recent success of these agonists, also known as psychedelics, like psilocybin for the treatment of anxiety, depression, obsessive-compulsive disorder (OCD), and addiction, has ushered in a renaissance in the way these compounds are perceived in the medical community and populace at large. One emerging therapeutic area that holds significant promise is their use as anti-inflammatory agents. Activation of 5-HT2A receptors produces potent anti-inflammatory effects in animal models of human inflammatory disorders at sub-behavioural levels. This review discusses the role of the 5-HT2A receptor in the inflammatory response, as well as highlight studies using the 5-HT2A agonist (R)-DOI to treat inflammation in cellular and animal models. It also examines potential mechanisms by which 5-HT2A agonists produce their therapeutic effects. Overall, psychedelics regulate inflammatory pathways via novel mechanisms, and may represent a new and exciting treatment strategy for several inflammatory disorders.
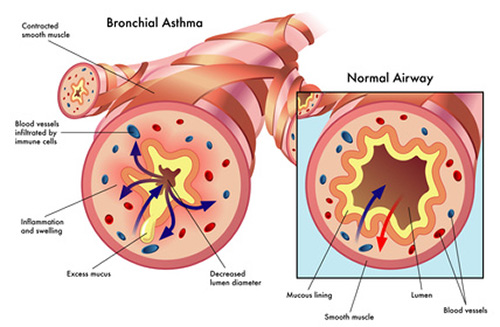
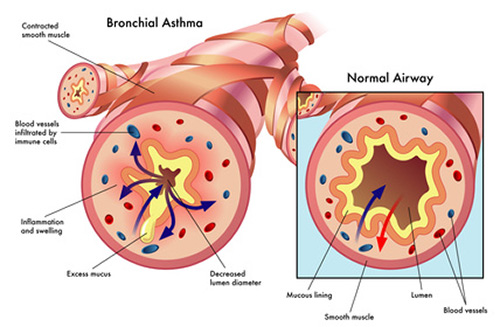
Psychedelics produce a potent blockade of the inflammation produced by TNF-α in cell and animal models of inflammation. Because of TNF-α’s controversial role in asthma and (R)-DOI’s impact on numerous factors contributing to the differentiation of multiple immune cells, we believe that the effects of 5-HT2A receptor activation likely extend far beyond the mere blockade of TNF-α signalling. Given the select nature by which (R)-DOI only blocks sub-sets of pro-inflammatory mediator expression, psychedelics may modulate histone modifications and epigenetic signalling for their therapeutic effects. In asthma, an interplay between the acetylation and de-acetylation states of histones in inflammatory genes has been well documented. Furthermore, histone de-acetylase (HDAC) inhibitors have been shown to reduce eosinophilic inflammation and AHR in mouse models of asthma. It is certainly plausible that 5-HT2A receptor activation modulates histone acetylation and methylation patterns to promote the expression of anti-inflammatory genes and repress the expression of pro-inflammatory genes. Only recently has it been establishes that 5-HT2A receptor activity can alter epigenetic factors.
The remaining questions regarding psychedelics and inflammation include:
1. Do 5-HT2A agonists have more pronounced effects in some cell types more than others?
2. Does 5-HT2A receptor activation modulate differentiation of immune-related cells to more anti-inflammatory phenotypes?
3. What are the effects of chronic administration of a 5-HT2A agonist in peripheral tissues to treat immune-related disorders?
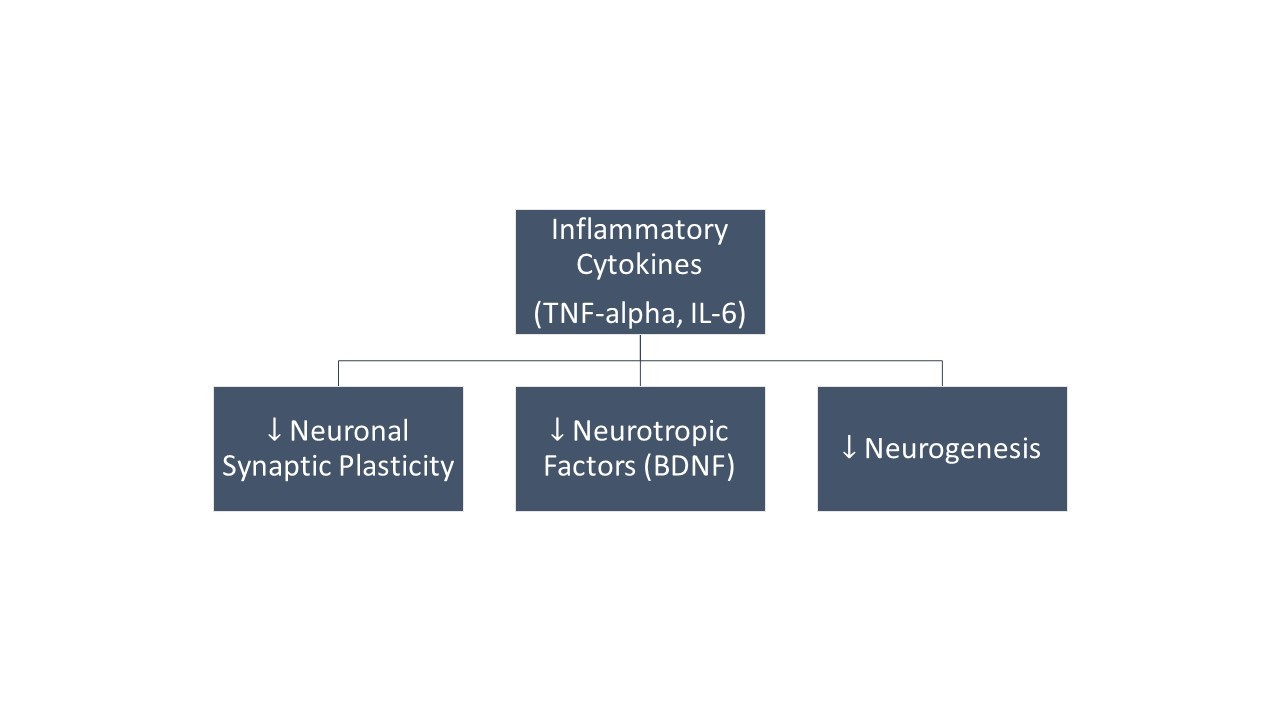
It is tempting to speculate on the nature of 5-HT2A receptor activation in other inflammatory disorders. Because 5-HT2A receptor activation impacts the expression of several key inflammatory mediators and the variety of effects we have observed in animal models of inflammation, we believe that psychedelics may be of therapeutic value to a wide range of inflammatory disorders in humans. With regard to therapeutic aspects of psychiatric disorders like depression, putative suppression of neuroinflammation by psychedelics may play a key role in the long-term stability of the reported anti-depressant effects after a single treatment. Another putative component may be stimulation of neurogenesis. For example, the psychotropic ingredient of the Amazonian tea ayahuasca can stimulate hippocampal neurogenesis, which has been shown to reduce depression-like behaviours.
Although the use of sub-behavioural levels of psychedelics remains to be validated as an effective therapeutic strategy for inflammation in humans, the data from cellular and animal models is promising, and these agents represent small molecule, highly bioavailable, inexpensive and steroid sparing treatments for inflammatory-related diseases like asthma, atherosclerosis, inflammatory bowel disease, and rheumatoid arthritis. One possible barrier to the development of psychedelics for use in the clinic is that the majority are scheduled and controlled substances in the United States and several other countries. Nevertheless, drugs that activate the 5-HT2A receptor and that have been shown to produce psychedelic effects in humans have already been FDA approved (e.g. lorcaserin). Although the results we discuss here are promising, more research is needed to fully unlock their therapeutic potentials, and to discover the molecular mechanisms underlying their effects.
*From the article here :

Psychedelics as anti-inflammatory agents - PubMed
Serotonin (5-hydroxytryptamine, 5-HT)<sub>2A</sub> receptor agonists have recently emerged as promising new treatment options for a variety of disorders. The recent success of these agonists, also known as psychedelics, like psilocybin for the treatment of anxiety, depression...
Last edited: