I have seen reaction specifics for the phophorylation of phenols, but yields were somewhere around 18%, and your stomach acid will immediately dephosphorylate it anyway. Psilocin (4-OH-DMT) has been found to be just as active per mole than Psilocybin (4-PO4-DMT). 4-MeO substitution leads to a decrease in activity (see TiHKAL).
There are other, older (read easier) ways to synth psilocin and DMT than are listed in TiHKAL. A common plant hormone, 3-indole acetic acid, can be reduced to a primary alcohol which can then by halogenated and traded for dimethylamine via a nucleophilic substitution reaction.
This reaction is one of the oldest methods of producing synthetic DMT but appears in ancient, hard to find journals and thus making looking up reaction specifics and choice of solvents more like real work. If you are going to go to that much trouble, make some LSD from LSA (from the hydrolysis of any number of prescription migraine drugs of the ergoline family) and thionyl chloride and diethylamine (from the hydrolysis of the insect repellant DEET). Go to
www.orgsyn.org and find all the reaction specifics for the conversion of a carboxylic acid to an N,N-diethylamide; one paper found there uses molecular sieves and strict anhydrous conditions to get about a 90% yield, stellar for the likes of LSD. However, the only FOAF who I have ever talked to who made LSD (1/2 gram) to pay for school did so at his place of work on a Saturday in the clean room. If you don't use lots of protective gear, then be prepared to trip harder than you probably ever have before. One gram of LSD tartrate equals 10,000 fairly strong hits.
Ibogaine really doesn't sound that great to me, and DMT crystals (crystallized from hexane with freebase DMT) can be obtained from the extraction and crystallization of DMT from the Mimosa hostilis tree roots and/or bark. Shrooms are also easily grown from kits; sterility is a must in this case. I have heard of fish tanks / aquariums and plenty of bateriocidal, non-toxic Clorox (sodium hypochlorite; NaOCl) being used to those ends.
As for the numbering system of indoles: look at a correct Kekule diagram of the structure of indole, with the 6-membered benzene ring on the left and the 5-membered ring with the amine on the right. Numbering of indole starts with that nitrogen being assigned number one and continues counterclockwise. Carbon numbers 2 and 3 are in the same ring as the nitrogen/amine. The reason the next carbon is skipped in the IUPAC numbering system is that that carbon already has 4 sigma bonds by necessity. Since no new functional group can bond to that carbon, there is no need to assign it a number. Continue numbering counterclockwise on the benzene ring. The last carbon you name should be #7. Excluding all hydrogens, indole contains 9 atoms (8 carbons and 1 nitrogen). Since two of the carbons that are members of both the 5 and the 6 membered ring have 4 sigma bonds already, can't bond to anything else and are left out of the numbering nomenclature, it is only correct that carbon #7 be the last to be numbered. 7 + 2 = 9.
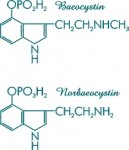
As for norbaeocystin and baeocystin, their 4-phosphoryloxy groups are so fragile, besides being totally unnecessary for psychoactivity, as to hydrolyze in any process which would add the necessary N-methyl or N,N-dimethyl groups to your aliphatic nitrogen. However, norbaeocystin without the phosphate could be prepared from the formylation of 4-OH-indole, followed by condensation with nitromethane and reducing any number of ways. From there, N,N-dimethylation to psilocin can be accomplished with formaldehyde, methanol and sodium cyanoborohydride. There is no way you are going to get any
4-OH-tryptamine from tryptamine, but you could easily make DMT from tryptamine. Tryptamine can be had from the reflux of the amino acid L-tryptophan in a high boiling solvent such as turpentine (I think) via a decarboxylation mechanism.
Interestingly, amphetamine side chains have been added onto every available carbon that indole has got. Substitution at the 3-indole position leads to AMT, which is a kind of good / kind of crappy drug. The only other psychoactive product was found to be 5-(2-aminoisopropyl) indole. That is MDA with a 3,4-phenyl
-CH=CH-NH= ring instead of the usual 3,4-phenyl -O-CH2-O- group. It is reported to be a stimulant by Shulgin, the renegade master. I have no idea how to synthesize this final curiousity. All formylations of indole that I know of will naturally add to the #3 position rather before the #5 position, but I'm sure there are ways around that problem. Lastly, you could try 4-OH-MIPT if you know where to look. Hope that helps.

 ) (which would hopefully add CH2CH2-NH2 at the 2- position.
) (which would hopefully add CH2CH2-NH2 at the 2- position.