Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
N&PD Moderators: Skorpio | thegreenhand
You should go for ketone when there are substitutions on the 2' positionSwissTargetPrediction
swisstargetprediction.ch
idk if I drew this right but ketamine without the ketone, or double bonded oxygen. 2-chloro-pcm?
SwissTargetPrediction
swisstargetprediction.ch
2-chloro-pce?
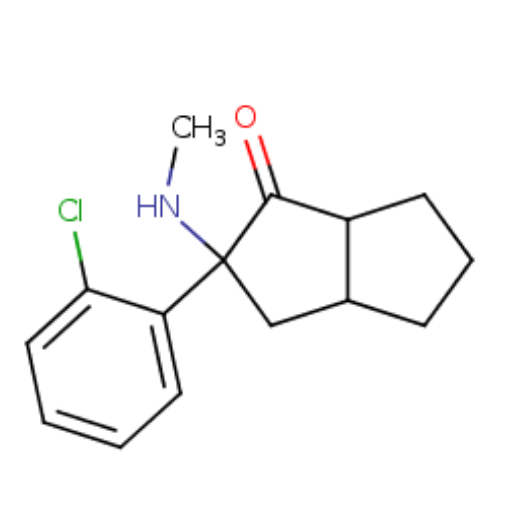
I don't think these are used in dentistry. Usually it's lidocaine and epinephrine, or maybe procaine used as anesthetics. But the more likely explanation is just inflammation from the trauma of a major dental surgery. I know when I had my wisdom teeth out the swelling and pain were very intense. They only gave me ketorolac too, which wasn't enough, let's just say....Is that what caused me to be unable to drink for 24h, let alone eat, after getting my wisdom teeth removed?
reasoning? the ketone is not required for activity, if anything it reduces the potency somewhat and shortens the half life, PCE has similar half life to PCP (super long) but methoxetamine and ketamine have much shorter onesYou should go for ketone when there are substitutions on the 2' position
I'm asking you the permission to post the two molecules I posted in Dresden's thread, to discuss the stp results where the chloro one has HERG but not the fluro one according to STP, and because I think these chems deserve to be posted here, or else I'll feel like this tread is dying
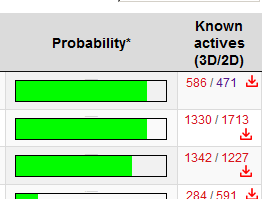
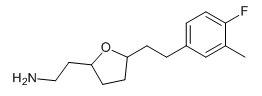
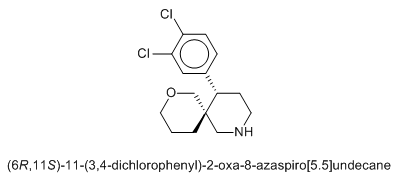
If I meet you in person I'm going to bonk you on the head with a rolled up newspaper. STP IS NOT AN ACCURATE PREDICTOR OF DRUG ACTIVITY. All it does is compare structures with known active compounds, there is no docking simulation of any sort done.STP helps to insure it's at least active.
How are you overlaying the structures? The only way that makes any sense is by comparing 3D structures (preferably in the active binding conformation, failing that, in energy minimized conformation). "Overlaying" 2d structures doesn't work too well because in reality, most molecules are not perfectly flat and planar.. And it overlays troparil just fine,
There's 3 chiral centres there, so 8 total isomers. Just something to consider. Cocaine has the same issue - of the 8 possible isomers only one is an effective stimulant.as it overlays just fine
Given that "fluoro-ephedrone" already refers to 4-fluoromethcathinone, and likewise "ephylone" already refers to N-ethylpentedrone, I don't think those are good names.Fluoro-Ephedrone or Fluoro-Ephylone
I don't mean to be negative, but these two molecules do not look promising.
Dopaminergic cannabinoid, shares androgen and estrogen liability. Should be euphoric.
Now let'd take the above and make it a bit more expensive, to get pretty much similar effects.. As of yet, a gold holding dopamine agonist etc etcSwissTargetPrediction
swisstargetprediction.ch
No they aren'tI don't mean to be negative, but these two molecules do not look promising.
I like the froggy. Just one little nitpick, the hashed bonds you draw should take the same shape as the wedges, and they should not have a gap at the endhere's my drawing.
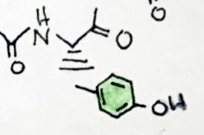
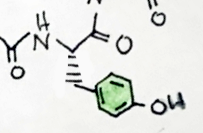
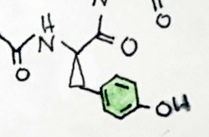
From what i've read it is anything but opioid like. I think Hamilton Morris did an episode where he took sapo, let's just say there was not a lot of blissful nodding off.The experience is described as opiate like.
What don't you understand? These aren't too difficult to answer I don't think.Thanks for the answer, the thing is I can't seem o understand your questions.. R-S most active? If you could explain it I'll learn from it, please?
Ît overlays troparil by the piperidine and tropane nd phenyl position in 2dI like the froggy. Just one little nitpick, the hashed bonds you draw should take the same shape as the wedges, and they should not have a gap at the end


The other way you can draw those bonds is as an unfilled version of the wedge bond, like this:

From what i've read it is anything but opioid like. I think Hamilton Morris did an episode where he took sapo, let's just say there was not a lot of blissful nodding off.
What don't you understand? These aren't too difficult to answer I don't think.
Question 1, Are you "overlaying" structures by making 3D models of them, or are you "overlaying" the 2D skeletal drawings?
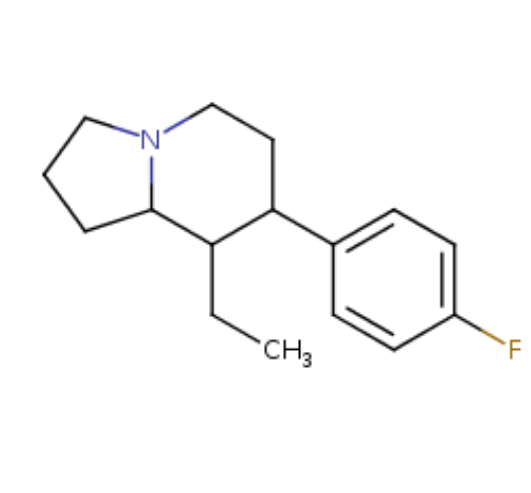
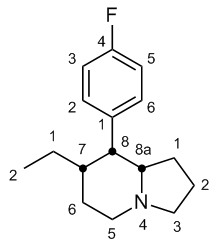
Question 2. Can you point out which carbon atoms are chiral centers on your indolizine? A chiral center is a carbon atom that has 4 different groups bonded to it.
(answer to question 2)
NSFW:
Chiral centers are marked: there are 3 sites - carbons 7, 8, and 8a
yeah I actually watched that episode! I like Hamilton's episodes. Yeah it doesn't look to pleasant. But I'm a connoisseur.. haha. Thank you for pointing out what I could do better (I haven't taken orgo yet...).I like the froggy. Just one little nitpick, the hashed bonds you draw should take the same shape as the wedges, and they should not have a gap at the end


The other way you can draw those bonds is as an unfilled version of the wedge bond, like this:

From what i've read it is anything but opioid like. I think Hamilton Morris did an episode where he took sapo, let's just say there was not a lot of blissful nodding off.
That's OK, neither have I. (seriously)(I haven't taken orgo yet...).
I'm a connoisseur of nicely drawn structuresBut I'm a connoisseur.. haha.
