-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
paracelsius
Bluelighter
- Joined
- Mar 11, 2020
- Messages
- 197
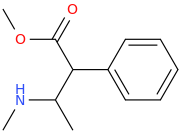
^ beta amino phenylacetates are unstable. tend to eliminate ammonia (or amine) very easily (even at ambient temperature) to give the styrene and amine. had a hard time isolating similar compounds in the past.unlike the phenydates tho!? but who knows with this particular compound?
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
The other possibility is that everyone assumes someone has already tested that compound in the 1950s or so, and found it useless. I couldn't find any original publication by the guy who made methylphenidate first time back then (and used his wife and himself as a guinea pig).
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
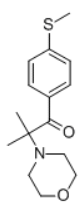
Irgacure 907, a UV photo-initiator for radical cross linking reactions. Looks like it'd be a cathinone stimulant of sorts if ingested.
4-methylthioamphetamine is a selective serotonin releaser according to Wikipedia. It could be that this compound doesn't release dopamine either. For some reason the STP online app thinks it could also bind to the GABA-A receptor. Some known direct GABA-A ligands have a heterocyclic ring with both a nitrogen and oxygen atom, maybe that's the reason.
blueberries
Bluelighter
- Joined
- Jan 13, 2011
- Messages
- 339
Dioxetamine
Since we're no longer allowed STPs, I should say it's an opioid with NMDA antagonism with high D2 and sigma agonism.
Last edited:
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
Piperonyl butoxide, a pesticide that looks like an MDMA precursor
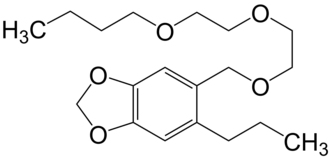
But if you try to halogenate it, the halogen will first go to the benzylic carbon, and you probably can't cleave the long ether tail without destroying the methylenedioxy structure in the process.

But if you try to halogenate it, the halogen will first go to the benzylic carbon, and you probably can't cleave the long ether tail without destroying the methylenedioxy structure in the process.
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
Fenazoxine, a cyclized derivative of orphenadrine, is said to be a stimulant and monoamine reuptake inhibitor without anticholinergic side-effects.
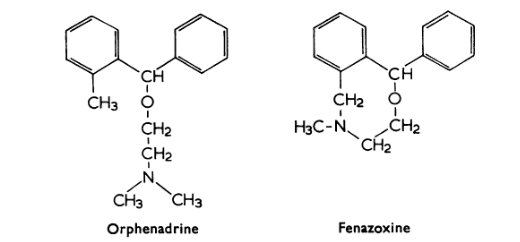
https://bpspubs.onlinelibrary.wiley.com/doi/pdf/10.1111/j.1476-5381.1969.tb09523.x

https://bpspubs.onlinelibrary.wiley.com/doi/pdf/10.1111/j.1476-5381.1969.tb09523.x
jose ribas da silva
Bluelighter
- Joined
- May 10, 2019
- Messages
- 3,888
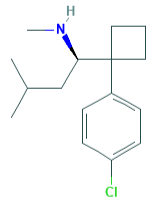
R-desmethyl-sibutramine reuptake inhibitor, 4nM at NET, 12 nM at DAT, 44 nM at SERT
R-didesmethyl-sibutramine, its metabolite, 13 nM NET, 8.9 nM DAT, 140 nM SERT
possible cocaine/methylphenidate type stimulant drug?
what the fuck is that a carbonic structure constrained by 90 degrees?
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
Oh, it has a new name. That structure also made me think of an orphenadrine with one of the N-methyls removed. It could be less of an anticholinergic compared to monoamine reuptake inhibition.
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
blueberries
Bluelighter
- Joined
- Jan 13, 2011
- Messages
- 339
PC-Adamantane (PCAd)
I have no idea how it would act though as STP hasn't got that far in it's capacity to understand it though...any ideas?
Or....
Ohhh; HOLY CRAP
I know we aren't allowed STP's here but oh my word:
Last edited:

R-desmethyl-sibutramine reuptake inhibitor, 4nM at NET, 12 nM at DAT, 44 nM at SERT
R-didesmethyl-sibutramine, its metabolite, 13 nM NET, 8.9 nM DAT, 140 nM SERT
possible cocaine/methylphenidate type stimulant drug?
The cyclobutyl moiety interested me to the extent that I wanted to add it to the MDMA scaffold - I believe they overlay well. Sibutramine itself has been taken off the market, hasn't it?
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
How small can you make the cycloalkyl ring in propylhexedrine and still retain activity? In some organic chemistry textbook I saw a cyclopentyl derivative that was said to be a stimulant, but I don't have that book anymore.

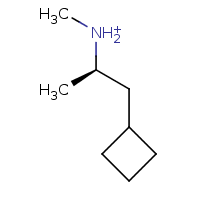
This was a good example of how the STP online app can produce misleading results. It thinks that cyclopentyl compound is mainly a nicotine receptor ligand and cholinesterase inhibitor, and only secondarily a dopamine reuptake inhibitor (it doesn't have this compound in the database as a known DAT ligand).


This was a good example of how the STP online app can produce misleading results. It thinks that cyclopentyl compound is mainly a nicotine receptor ligand and cholinesterase inhibitor, and only secondarily a dopamine reuptake inhibitor (it doesn't have this compound in the database as a known DAT ligand).
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
How small can you make the cycloalkyl ring in propylhexedrine and still retain activity?
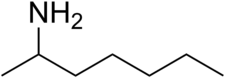
2-aminoheptane is a sympathomimetic, and you cannot really get any simpler than that, I don't think.
- Status
- Not open for further replies.