-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
Pawhuskin A is a cool compound, non-nitrogenous kappa receptor (mainly) blocker.
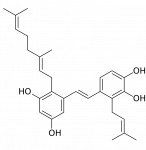
You could probably use that as an addition to a non-selective opioid agonist like the akuamma alkaloids, which bind more to kappa than mu receptors and therefore aren't very euphoric by themselves. Much like the "blue velvet" mixture of pentazocine and an antihistamine (I guess that mixture works because the sedative antihistamine potentiates only the mu agonist effect and not the kappa).
Edit: someone with any level of actual physical dependence to opioids probably shouldn't try balancing like that, tho...

You could probably use that as an addition to a non-selective opioid agonist like the akuamma alkaloids, which bind more to kappa than mu receptors and therefore aren't very euphoric by themselves. Much like the "blue velvet" mixture of pentazocine and an antihistamine (I guess that mixture works because the sedative antihistamine potentiates only the mu agonist effect and not the kappa).
Edit: someone with any level of actual physical dependence to opioids probably shouldn't try balancing like that, tho...
Last edited:
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
The 4-amino version of methylphenidate is significantly more potent than MPH itself, but can't know if it has aniline-like toxicity...
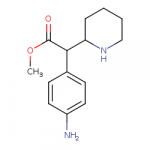
The SwissTargetPrediction also gives it a slim chance of binding to mu receptor.
If someone were to make a non-amine MPH with the piperidine nitrogen removed or replaced with oxygen, it would be easier to purify if it had an amino group on the benzene ring.
Edit1: Also, making it a 4-aminoethylphenidate gives it a little bit more likelihood of binding to mu receptor, similar to meperidine SAR.
Edit2: And, by reducing the shit out of antibiotic chloramphenicol, you could obtain an equivalent 4-amino version of amphetamine.

The SwissTargetPrediction also gives it a slim chance of binding to mu receptor.
If someone were to make a non-amine MPH with the piperidine nitrogen removed or replaced with oxygen, it would be easier to purify if it had an amino group on the benzene ring.
Edit1: Also, making it a 4-aminoethylphenidate gives it a little bit more likelihood of binding to mu receptor, similar to meperidine SAR.
Edit2: And, by reducing the shit out of antibiotic chloramphenicol, you could obtain an equivalent 4-amino version of amphetamine.
Last edited:
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
The essential oil of aniba canelilla tree is unusual in containing a nitro compound, 1-nitro-2-phenylethane... That could be used in producing alpha-benzylphenethylamine, but unfortunately that compound doesn't seem to be an amphetamine-like stimulant.

 www.sciencedirect.com
www.sciencedirect.com

Pharmacological characterization of BNMPA (α-benzyl-N-methylphenethylamine), an impurity of illicit methamphetamine synthesis
α-Benzyl-N-methylphenethylamine (BNMPA), an impurity of illicit methamphetamine synthesis, has previously been reported to produce convulsions in mice…

polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
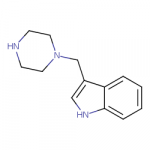
Some new compounds here (the first and the third image):
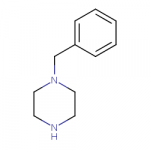
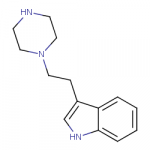
 www.swisstargetprediction.ch
www.swisstargetprediction.ch
 www.swisstargetprediction.ch
www.swisstargetprediction.ch
 www.swisstargetprediction.ch
www.swisstargetprediction.ch
For some reason the Swiss Target Prediction thinks that the N-(indolylethyl)piperazine is a better dopamine reuptake inhibitor than either N-benzylpiperazine or N-(indolylmethyl)piperazine.



SwissTargetPrediction
SwissTargetPrediction
SwissTargetPrediction
For some reason the Swiss Target Prediction thinks that the N-(indolylethyl)piperazine is a better dopamine reuptake inhibitor than either N-benzylpiperazine or N-(indolylmethyl)piperazine.
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
3,4-dichloropemoline
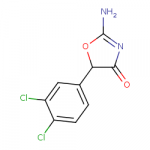
 www.swisstargetprediction.ch
www.swisstargetprediction.ch
Nothing seems to be known about this. If its dosage in mg is like 10 times less that that of pemoline, it could have less liver toxicity, but that advantage disappears with stimulant tolerance.

SwissTargetPrediction
Nothing seems to be known about this. If its dosage in mg is like 10 times less that that of pemoline, it could have less liver toxicity, but that advantage disappears with stimulant tolerance.
blueberries
Bluelighter
- Joined
- Jan 13, 2011
- Messages
- 339
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
The 4-amino version of methylphenidate is significantly more potent than MPH itself, but can't know if it has aniline-like toxicity...
View attachment 17784
The SwissTargetPrediction also gives it a slim chance of binding to mu receptor.
If someone were to make a non-amine MPH with the piperidine nitrogen removed or replaced with oxygen, it would be easier to purify if it had an amino group on the benzene ring.
Edit1: Also, making it a 4-aminoethylphenidate gives it a little bit more likelihood of binding to mu receptor, similar to meperidine SAR.
Edit2: And, by reducing the shit out of antibiotic chloramphenicol, you could obtain an equivalent 4-amino version of amphetamine.
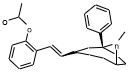
I've always mused about near methylphenidates that were also near fentanyls.
...
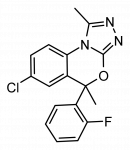
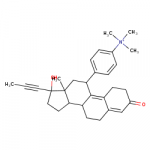
Wishing I had the specs to this one below, tried against the phenyltropanes.
Note the C1 position substitution, not a mistake, though maybe add a C2 position carbmethoxy
Attachments
Last edited:
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
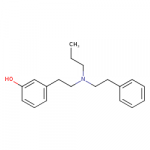
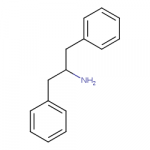
I've always mused about near methylphenidates that were also near fentanyls.
I drew some amine compounds like this in another thread:
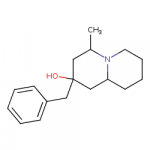
It's a possible dopamine reuptake inhibitor and also looks a bit like fentanyl or meperidine, and a pharmacology app predicts it's a possible mu receptor ligand.
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
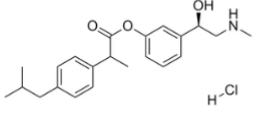
I drew some amine compounds like this in another thread:
View attachment 21584
It's a possible dopamine reuptake inhibitor and also looks a bit like fentanyl or meperidine, and a pharmacology app predicts it's a possible mu receptor ligand.
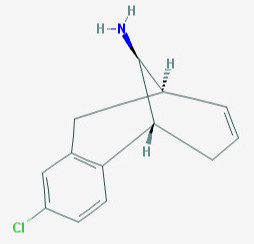
Reminds me of Org 6582:
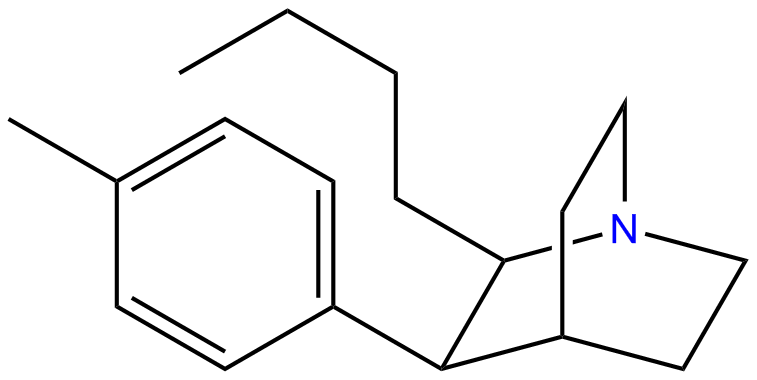
or Butyltolylquinuclidine:
Really they all turn out to be Zoloft analogues, more or less.
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
"cannabinosides", glycosylated cannabinoids
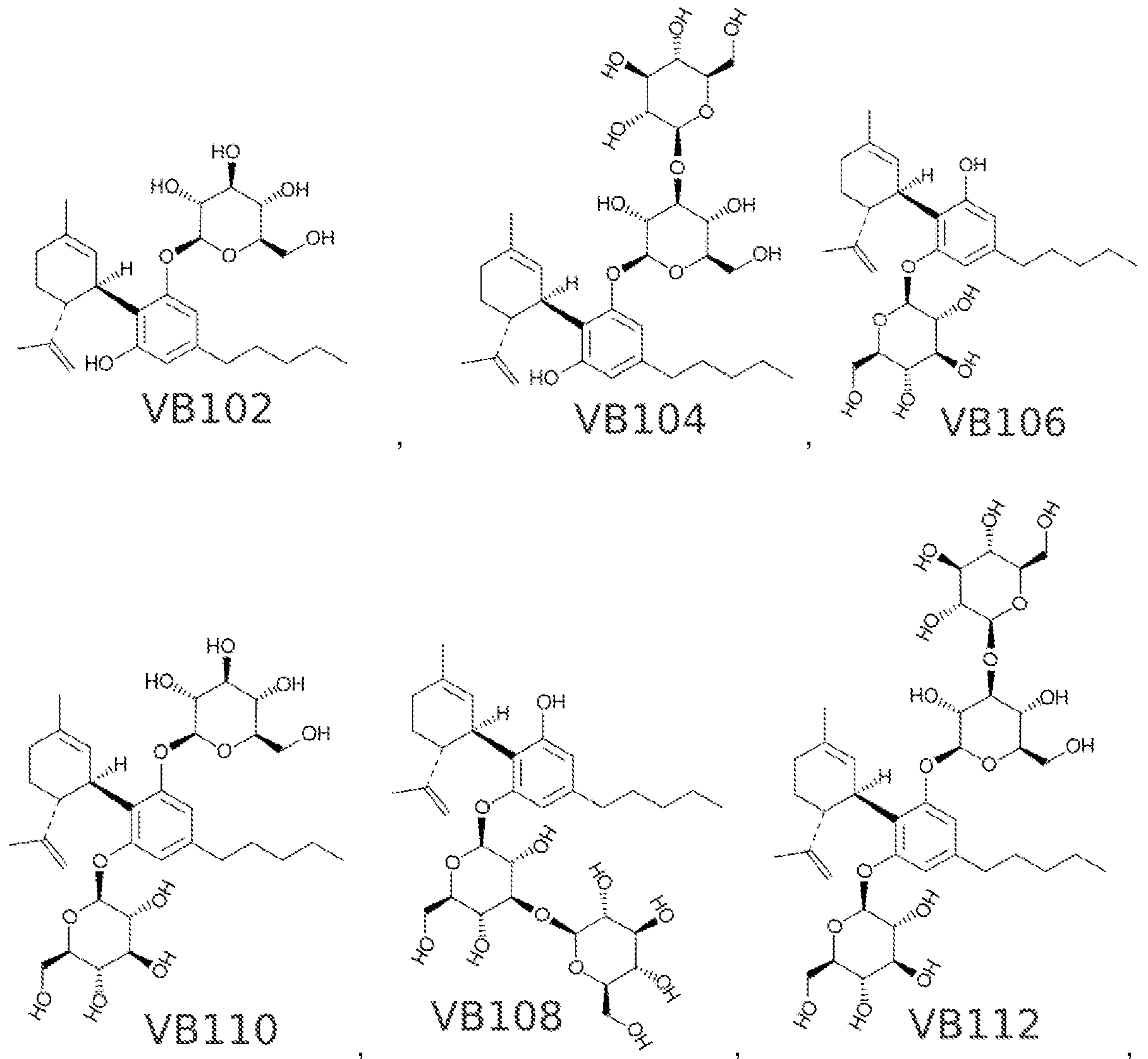
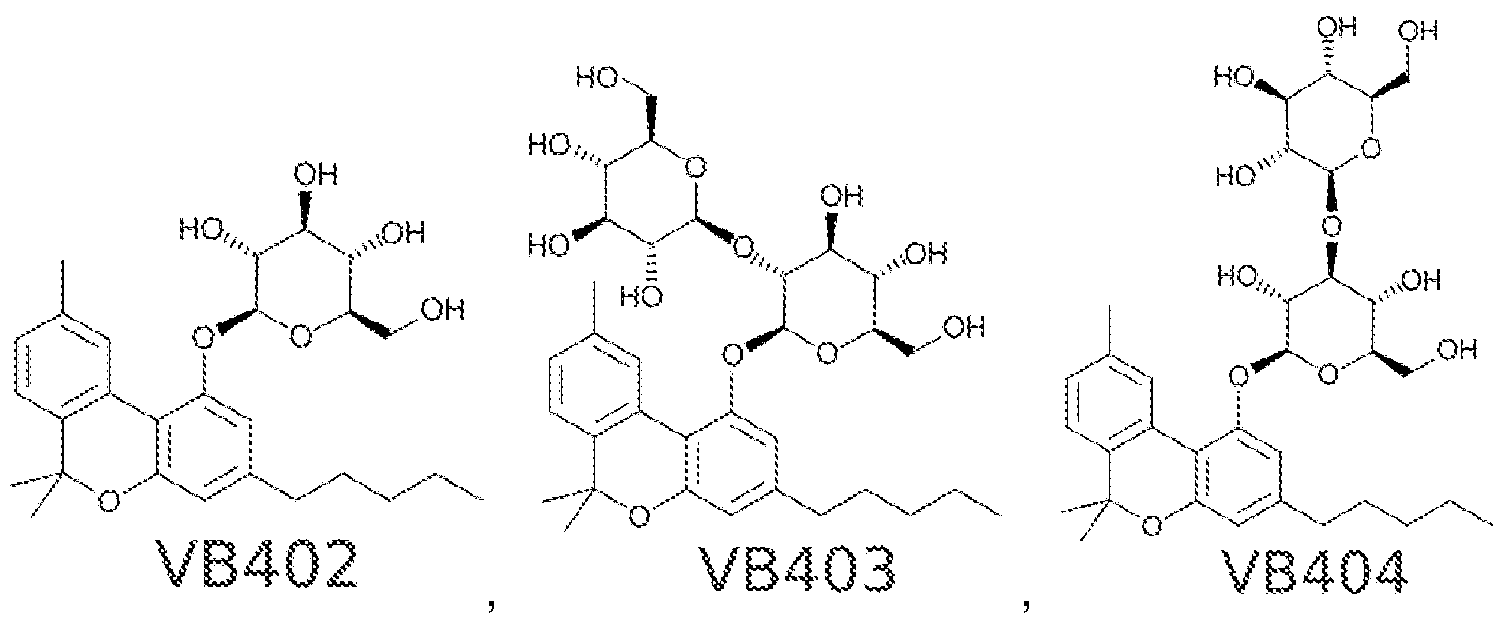
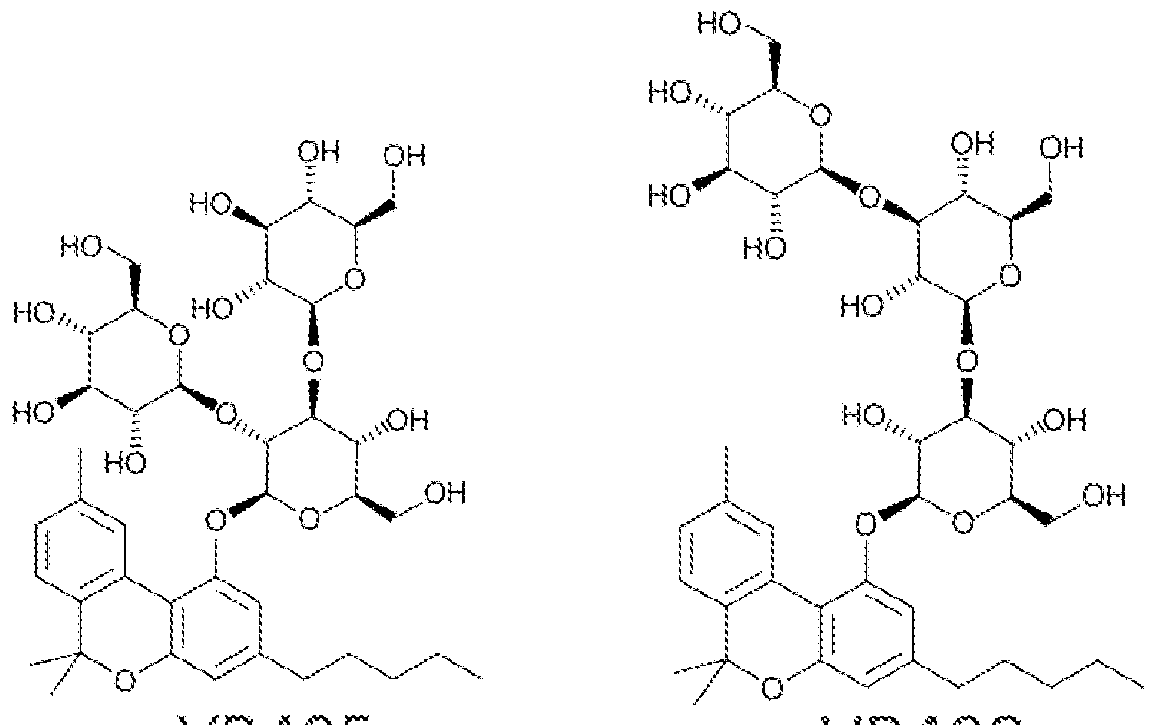
NSFW:



S.J.B.
Bluelight Crew
- Joined
- Jan 22, 2011
- Messages
- 6,886
Sweet!https://patents.google.com/patent/WO2017053574A1/en "cannabinosides", glycosylated cannabinoids
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
Reminds me of Org 6582:

or Butyltolylquinuclidine:

Those have a quite different connectivity, because the shortest distance between phenyl and nitrogen is only two carbon atoms. The compound in my post is more like 4-benzylpiperidine.
blueberries
Bluelighter
- Joined
- Jan 13, 2011
- Messages
- 339
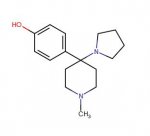
No-one want to to comment on my "Hydrolicyclomethylamine" ??
**Got the nomenclature wrong in the first!**
**Got the nomenclature wrong in the first!**
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
Those have a quite different connectivity, because the shortest distance between phenyl and nitrogen is only two carbon atoms. The compound in my post is more like 4-benzylpiperidine.
Still reminds me of them. 4-BP is like a once lengthened phenmetrazine
P.S. would somebody direct me to a on phone free molecule renderer
Last edited:
- Status
- Not open for further replies.