-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
blueberries
Bluelighter
- Joined
- Jan 13, 2011
- Messages
- 339
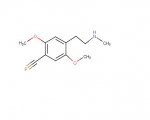
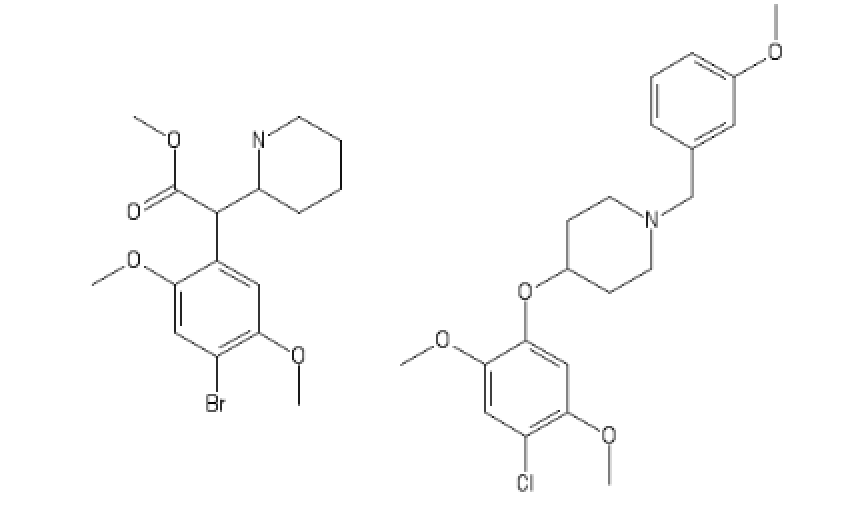
I call this one DOP!
Nothing to do with opiates but it could use a little work with nomenclature!
PS: I realise that some habits can't be helped but I really wouldn't mind finding out the structure!
Nothing to do with opiates but it could use a little work with nomenclature!
No offence but I can hardly understand what's going on here, could you make this a flat drawing so I can figure it out?!
PS: I realise that some habits can't be helped but I really wouldn't mind finding out the structure!
Attachments
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
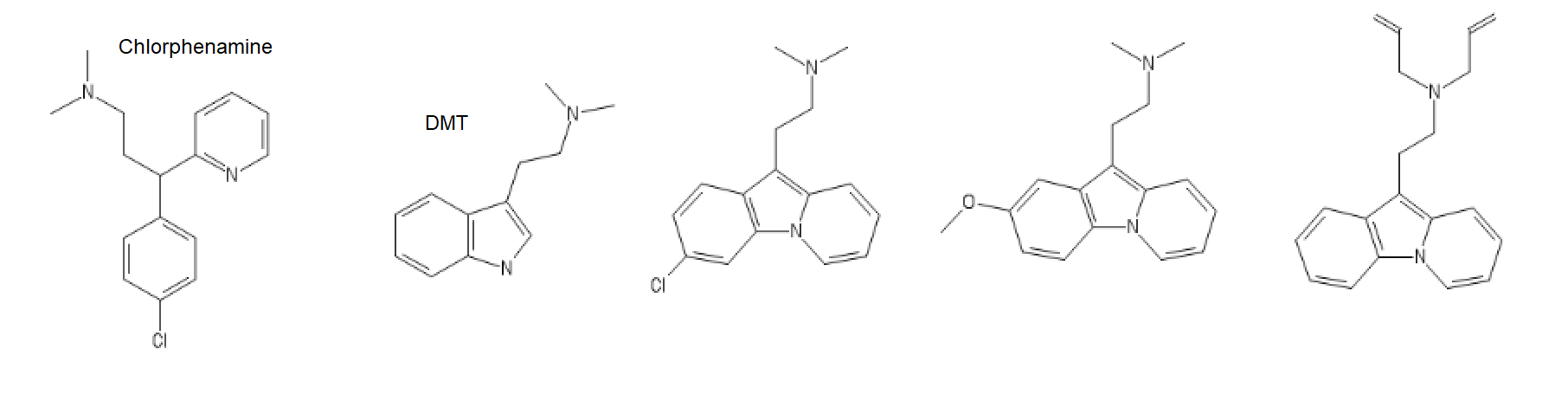
With DMT being investigated to suppress "voices" in Skizo patients and H1 being a general antipsychotic, maybe these could be antidepressive antipsychotics, give me a try I'd say! with chlorphenamine being well supported as a downer (sold with Ephedrine in France), it's widely used as a somniferum.
Would mixing and heating too much methanol with tyrosine make 4-methoxy Methoxyphenylalanine? Tyrosine on itsef is a bit boring.

Would mixing and heating too much methanol with tyrosine make 4-methoxy Methoxyphenylalanine? Tyrosine on itsef is a bit boring.
Last edited:
atara
Bluelighter
- Joined
- Apr 1, 2010
- Messages
- 2,785
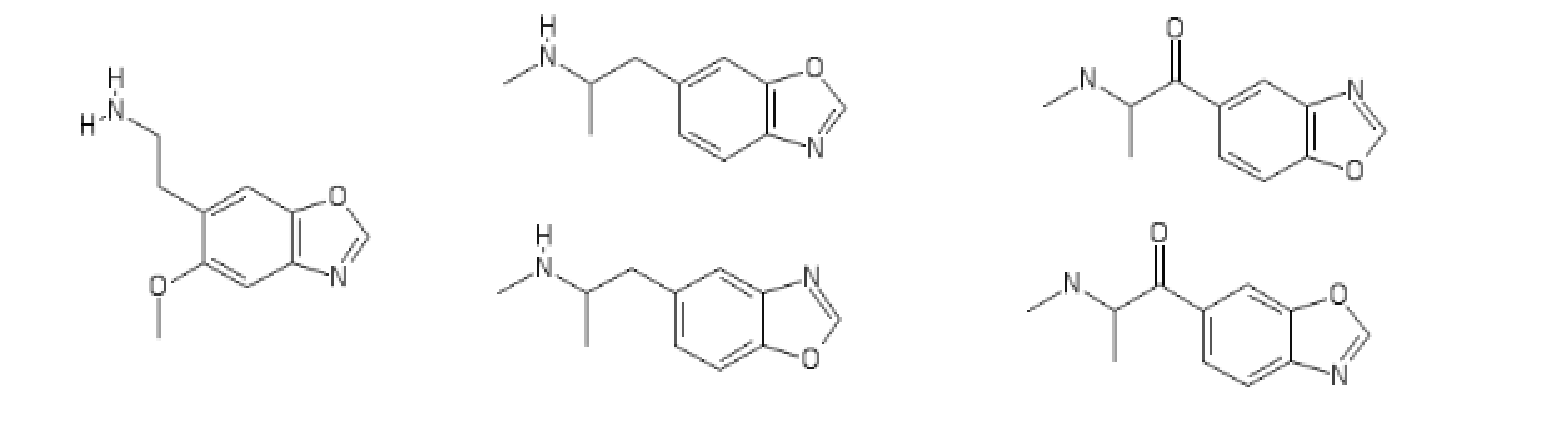
Have been away. Was wondering if this one ever popped up on the scene?
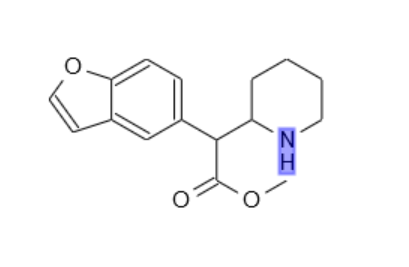
I think it's got a better chance of being a triple inhibitor than the methylenedioxy version (cf. analogy naphyrone:HDMP-28::MDPV:methylenedioxymethylphenidate)
blueberries: you get no points if you draw a triple-bonded phosphorus

I think it's got a better chance of being a triple inhibitor than the methylenedioxy version (cf. analogy naphyrone:HDMP-28::MDPV:methylenedioxymethylphenidate)
blueberries: you get no points if you draw a triple-bonded phosphorus
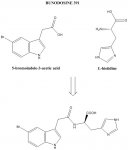
BUNODOSINE 391
Isolated from a sea anemone, this N-acylamino acid is a potent analgesic. Its effects are not reversed by naloxone but are reversed by serotonin receptor antagonists. Interesting lead for discovery work in the area of non-opioid analgesics. It would be interesting to evaluate the effects of different substituents on the indole & histidine nuclei. The occurence of this molecule in such an early life form may indicate a basic evolutionary role in nerve function.
It is interesting to note that 5-brominated tryptophan residues are found in so-called 'sleep peptides' produced by some cone snail species. These are 'post-translational' enzymatic modifications of the tryptophan residues in these peptides, the enzyme involved being a bromoperoxidase & the reaction mechanism being free-radical in nature. The key raw material is bromide ion, which is abundant in seawater.
Isolated from a sea anemone, this N-acylamino acid is a potent analgesic. Its effects are not reversed by naloxone but are reversed by serotonin receptor antagonists. Interesting lead for discovery work in the area of non-opioid analgesics. It would be interesting to evaluate the effects of different substituents on the indole & histidine nuclei. The occurence of this molecule in such an early life form may indicate a basic evolutionary role in nerve function.
It is interesting to note that 5-brominated tryptophan residues are found in so-called 'sleep peptides' produced by some cone snail species. These are 'post-translational' enzymatic modifications of the tryptophan residues in these peptides, the enzyme involved being a bromoperoxidase & the reaction mechanism being free-radical in nature. The key raw material is bromide ion, which is abundant in seawater.
Attachments
Last edited:
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
Ephylone compared to Pentylone is miles away, in a good way.
New atypical antipsychotic
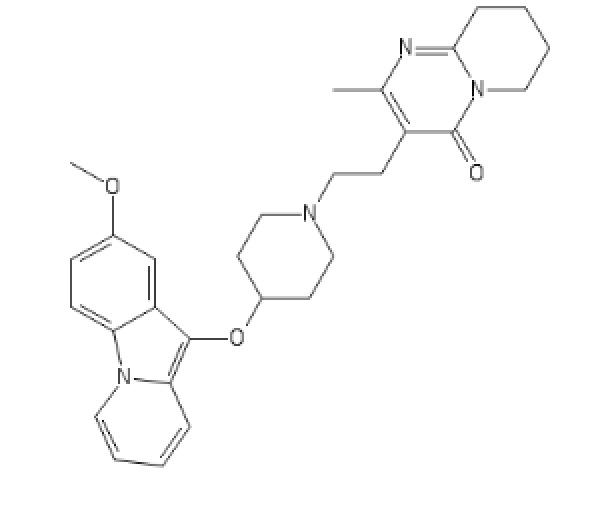
And to make the treated patient's life easier, some undead cathinones, because yes, being on stims and antipsychoticum at the same time is just better.
And if China keeps banning chems one by one every two years, I might live to see my little babys.
(I'd personnaly choose the 4-MethoxyHexen over the 4-MEE-CATH). 2F-EPD is for studying or everyday adderall replacement.
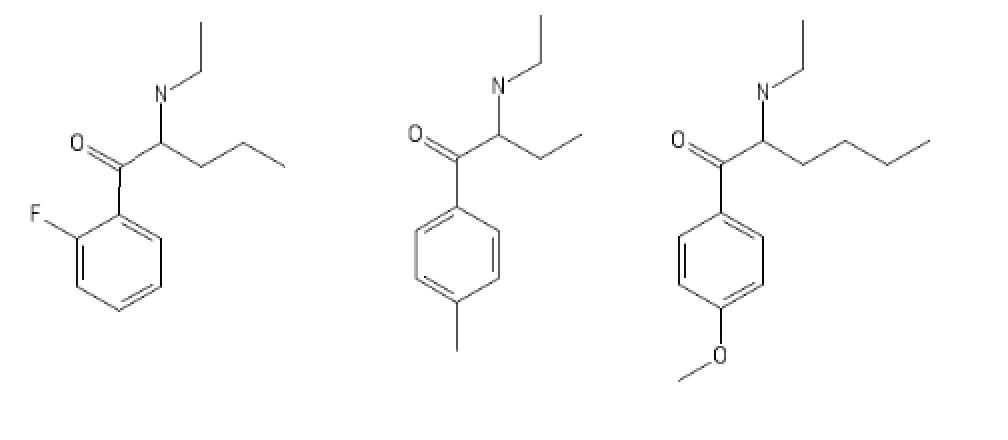
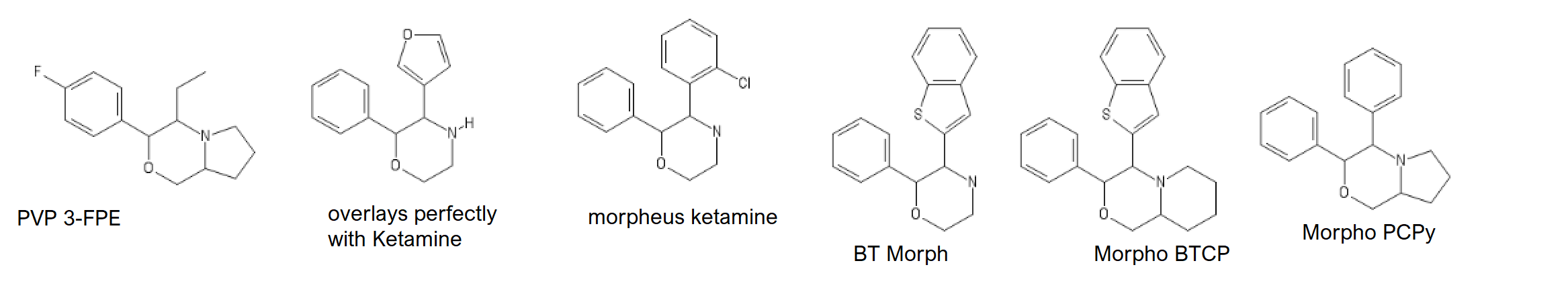
And for the 3-FPM lovers:
2-(3-fluorophenyl)-3-ethylmorpholine AKA 3-FPE
3-ethyl-2-phenylmorpholine AKA PE
3-ethyl-2-(3'-4'-Furan-4"-yl)-phenylmorpholine AKA FUPE
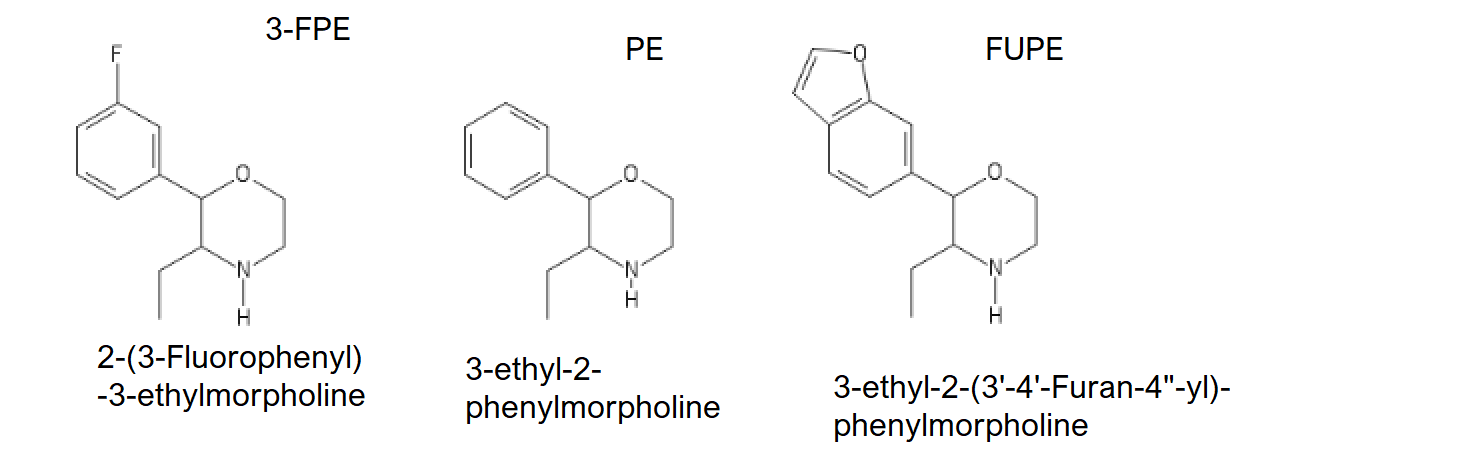
God do I want to try my chems.
FUPE's IUPAC might be wrong.
Morpheus
I'm so good a this
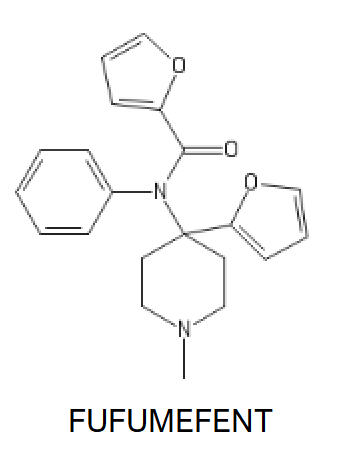
FUFUF:
New atypical antipsychotic

And to make the treated patient's life easier, some undead cathinones, because yes, being on stims and antipsychoticum at the same time is just better.
And if China keeps banning chems one by one every two years, I might live to see my little babys.
(I'd personnaly choose the 4-MethoxyHexen over the 4-MEE-CATH). 2F-EPD is for studying or everyday adderall replacement.

And for the 3-FPM lovers:
2-(3-fluorophenyl)-3-ethylmorpholine AKA 3-FPE
3-ethyl-2-phenylmorpholine AKA PE
3-ethyl-2-(3'-4'-Furan-4"-yl)-phenylmorpholine AKA FUPE

God do I want to try my chems.
FUPE's IUPAC might be wrong.
Morpheus

I'm so good a this
FUFUF:

Last edited:
^ Has anyone ever tested compounds where the n-phenethyl of fentanyl is replaced with n-methyl, or where the n-methyl of meperidine is replaced with n-phenethyl (or n-phenylisopropyl) group ?
They actually did do that with carfentanil.
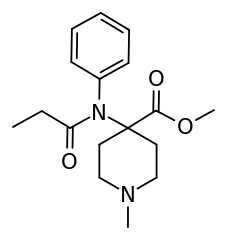
N-methyl-N-nor-carfentanil is roughly as potent as morphine. Considering that carfentanil is several thousand times the potency of morphine, this does not suggest that doing this to regular fentanyl (which is, after all, approximately 1% as potent as carfentanil) would result in a particularly impressive compound.
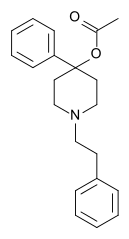
The N-phenethyl,N-nor-analogue of meperidine was also made... well, almost: PEPAP (PhenEthylPhenylAcetoxyPiperidine) also shortens the propionyloxy group to an acetoxy; at any rate, it is roughly 6-7 times as potent as morphine.
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
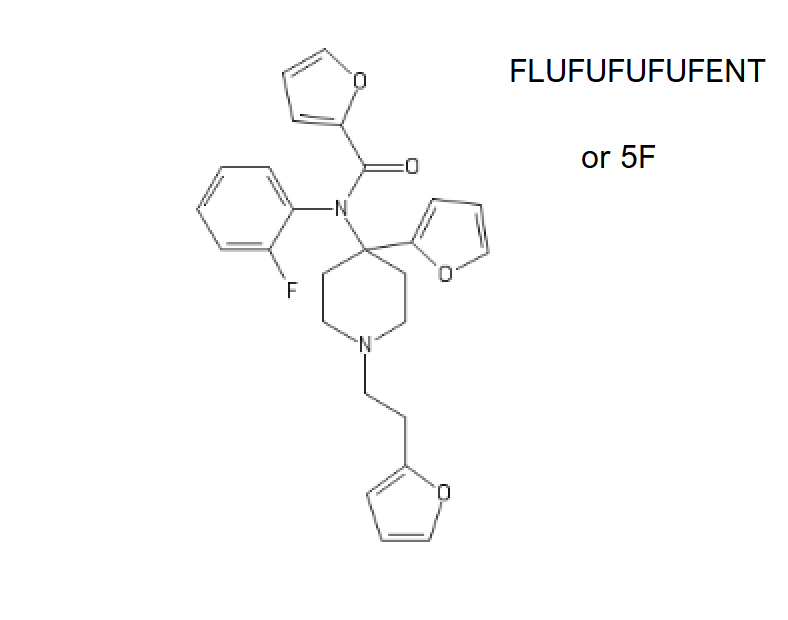
Safety Reason. But here's the Dangerous one, FLUFUFUFUFENT, AKA 5F, a piece of masterpork (yes you read that right).
I'd love a virtual reality where my brain would be remade molecule by molecule, in which every one of my drugs could be tested in the virtual brain. It will be a virtual Nirvana to it, yet feel so really unreal because it's so good.
I'm betting this could be a game in 30-40 years. Create the most pleasured brain. (Music sounds and body "ressentis" will of course be a big part of it")
In some words, life.
This should've been my 100st post.

I'd love a virtual reality where my brain would be remade molecule by molecule, in which every one of my drugs could be tested in the virtual brain. It will be a virtual Nirvana to it, yet feel so really unreal because it's so good.
I'm betting this could be a game in 30-40 years. Create the most pleasured brain. (Music sounds and body "ressentis" will of course be a big part of it")
In some words, life.
This should've been my 100st post.
Last edited:
Minor correction to my post above: I just realized I was confusing the structure of meperidine with that of MPPP.
Meperidine does not contain a propionyloxy group; it contains an ethyl carboxylate group, of which PEPAP's acetoxy group is the reverse ester.
Meperidine does not contain a propionyloxy group; it contains an ethyl carboxylate group, of which PEPAP's acetoxy group is the reverse ester.
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
There's a website where you can just throw a chem at it and it gives you an idea about the SAR activity; it's called swiss target prediction, I asked for Something like it some while ago, I think I'm going to post a thread with some of my bestest chems and a link to their SAR. Anyway more seriously, carfentanyl SARs http://www.swisstargetprediction.ch/result.php?job=1777385148&organism=Homo_sapiens
compared to: http://www.swisstargetprediction.ch/result.php?job=1428025724&organism=Homo_sapiens and http://www.swisstargetprediction.ch/result.php?job=1558530886&organism=Homo_sapiens that is stronger than carfent (!)
compared to: http://www.swisstargetprediction.ch/result.php?job=1428025724&organism=Homo_sapiens and http://www.swisstargetprediction.ch/result.php?job=1558530886&organism=Homo_sapiens that is stronger than carfent (!)
Gaffy
Bluelighter
- Joined
- Oct 27, 2018
- Messages
- 1,210
Here I'll be posting my best inventions, selected for their binding profiles.
It'll take some time to finish this thread (if I ever do as I've litterally thousands of chemicals to sort out and run through, and test with the SAR machine), but I'll post what I think deserves to be posted.
We'll start with Ketamine and Ephenidine analogs I've invented, and switch to BTCP analogs which will permit us to switch to DRIs: (And from there it'll be pretty random, as I can't classify all my compounds)
Ketamine
PCP
Ephenidine
Seven ringed Ketamine,
CycloHeptylKetamine : C1(C(CCCCC1)=O)(C2=CC=CC=C2)N(C)[H]
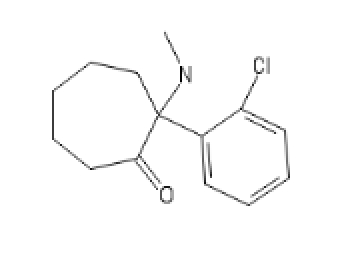
Seven ringed Methoxetamine,
CycloHeptylMethoxetamine: C1C(C(CCCC1)(C2=CC=CC(=C2)OC)NCC)=O
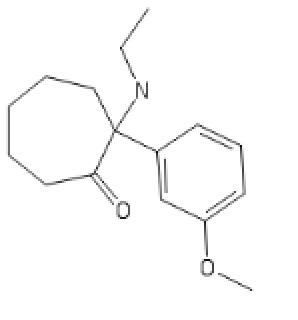
Adding a benzene group to the cycloheptyl:
3-MeO-PCPy with a PhenylCycloHEptyl ring: C1CC(CCC2=C1C=CC=C2)(C3=CC=CC(=C3)OC)N4CCCC4
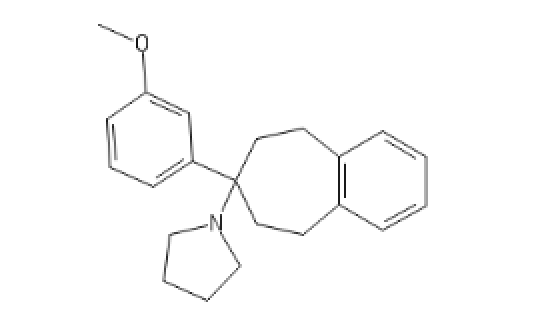
a little extra
(With very interesting SARs)
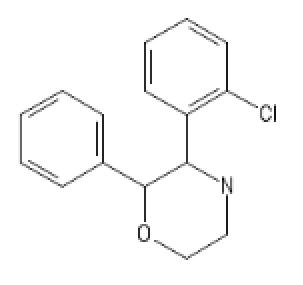
MorphoKetamine: ClC1=C(C=CC=C1)C1NCCOC1C1=CC=CC=C1
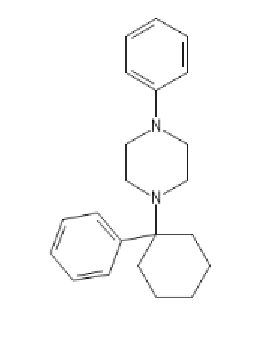
PCP with a N-PhenylPiperazine
PCPP: C1=CC=C(C=C1)N2CCN(CC2)C3(CCCCC3)C4=CC=CC=C4
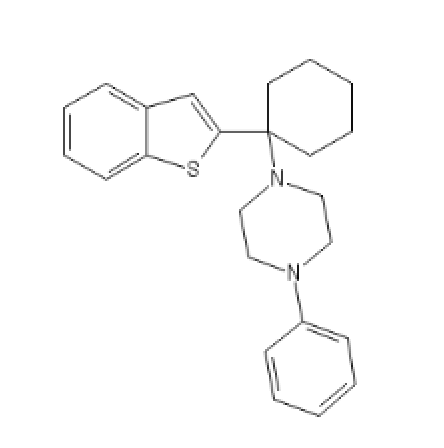
BTCPP, BTCP with a phenylpiperazine
BTCPP: C1=CC=C(C=C1)N2CCN(CC2)C3(CCCCC3)C5=CC4=CC=CC=C4S5
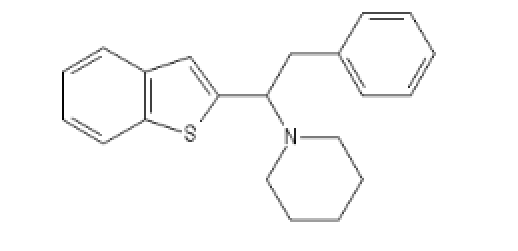
Three analogs between Ephenidine and BTCP:
BTPhenEthylPiperidine : C(C(N1CCCCC1)C1=CC2=C(S1)C=CC=C2)C1=CC=CC=C1
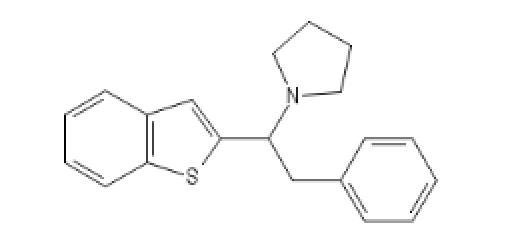
BTPhenEthylPyrrolidine: C12=C(C=CC=C1)SC(=C2)C(CC3=CC=CC=C3)N4CCCC4
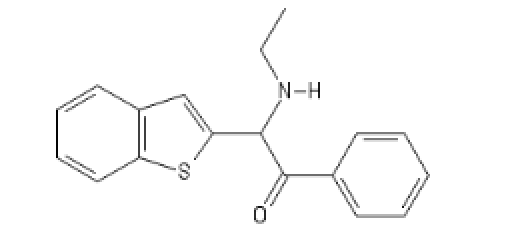
BTBKPhenEthylEthylamine: C12=C(C=CC=C1)SC(=C2)C(C(C3=CC=CC=C3)=O)N(CC)[H]
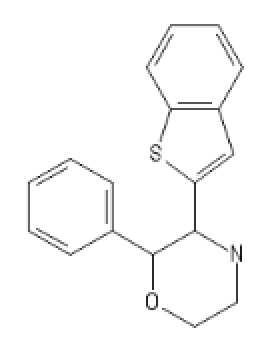
The morpholine analog of ketamine applied to BTCP:
MorphoBTCE: C1COC(C(N1)C1=CC2=C(S1)C=CC=C2)C1=CC=CC=C1
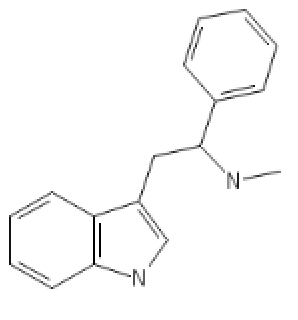
Mono-methylated alpha-phenylated tryptamine, a SNDRI that stands out:
N-Methyl-Alpha-Phenyl-Tryptamine: C1=CC=CC2=C1C(=C[N]2)CC(C3=CC=CC=C3)NC
Its N-Pyrrolidino analog
5-MeO-AMT
C1(=CC=C2C(=C1)C(=C[N]2)CC(N([H])[H])C)O
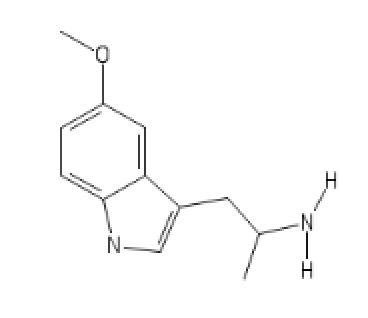
Cocaïne analogs: probably my best inventions
I Don't know what to call these, but they're wonderously promising
COC(=O)C(CC1=CC=CC=C1)C1CCCN1
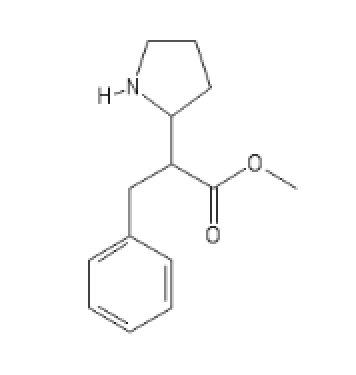
The N-Methyl, even more promising
C1(=CC=CC=C1)CC(C(=O)OC)C2CCCN2C
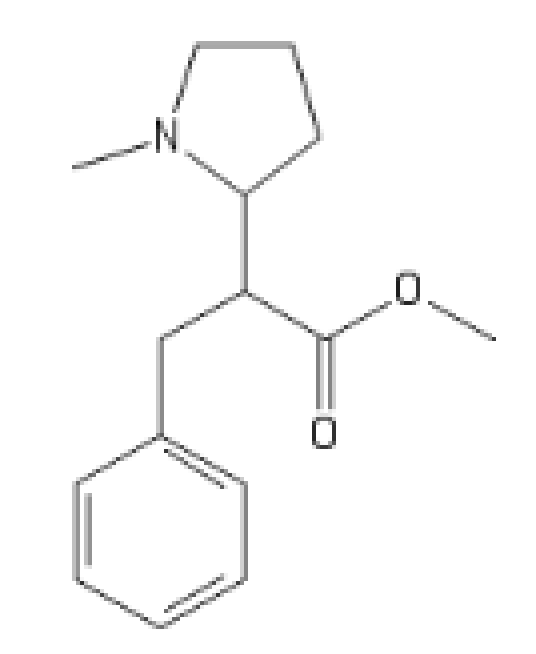
The local anaesthetic version, it's got high HERG activity:
C1=CC=CC=C1C(OCC(C(OC)=O)C2CCCN2[H])=O
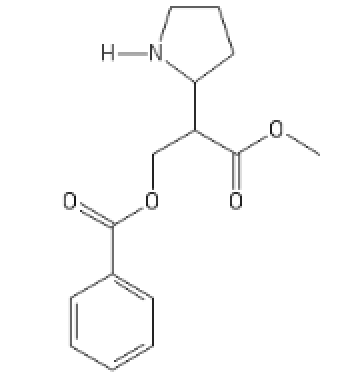
The N-Methyl version, with even more HERG activity, which makes it cardiotoxic
COC(=O)C(COC(=O)C1=CC=CC=C1)C1CCCN1C
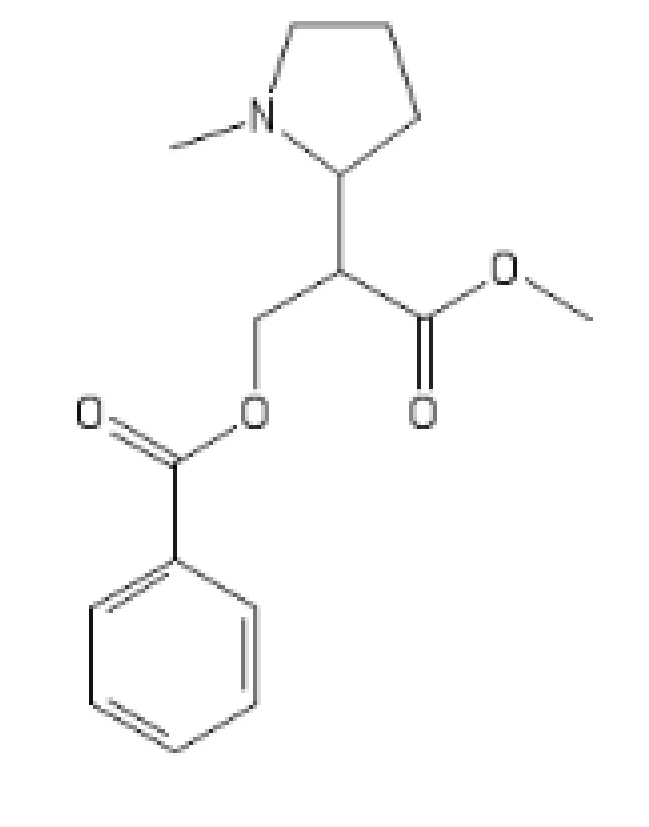
An outstanding one,both SNDRI and MOR (!)
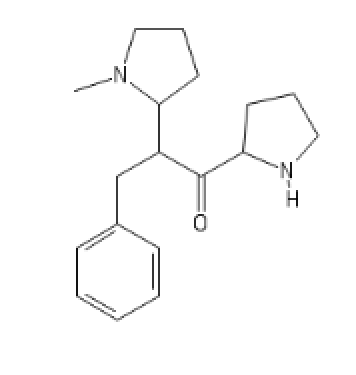
N'-N-Methyl version
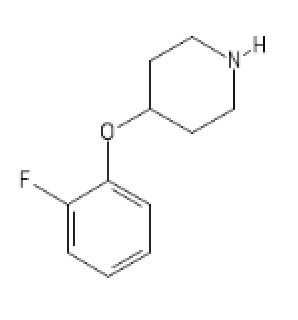
2-FA Light
C1(=CC=CC=C1OC2CCN(CC2)[H])F
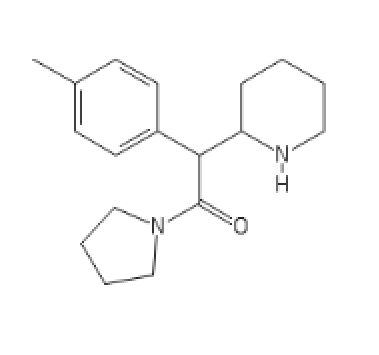
4-MethylMethylphenidate N-pyrrolidine analog
C1=CC=CC=C1C(C(N2CCCC2)=O)C3CCCCN3[H]
PVP Methylphenidate: Surprisingly good
C1=CC=CC=C1C(C2CCCC3CCCN23)C(OC)=O
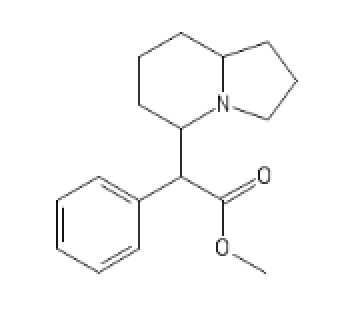
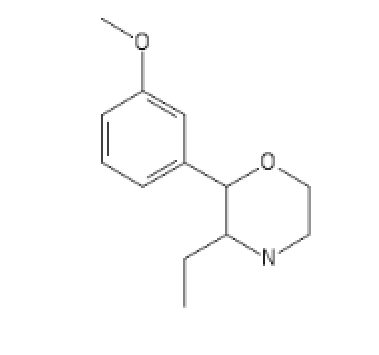
N-pyrrolidino Version
3-MeO-PhenEtrazine
C1=C(C=CC=C1C2C(CC)N(CCO2)[H])OC
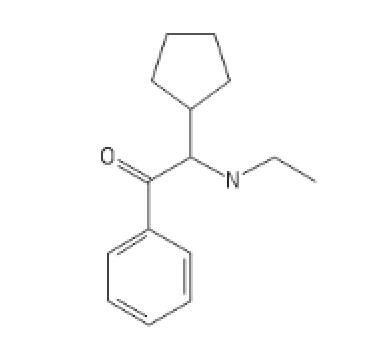
CyclopentylEthylCathinone
CCNC(C1CCCC1)C(=O)C1=CC=CC=C1
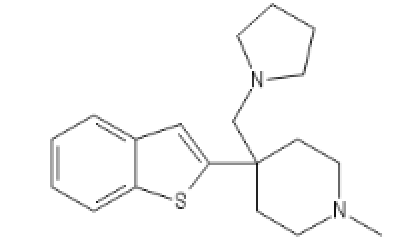
Two Ketobemidone variants, go guess how I guessed
BTKETO1
C1=CC=C2C(=C1)C=C(S2)C3(CCN(CC3)C)CN4CCCC4
BTKETO2
C1=CC=C2C(=C1)C=C(S2)C3(CCN(CC3)C)C(N4CCCC4)=O
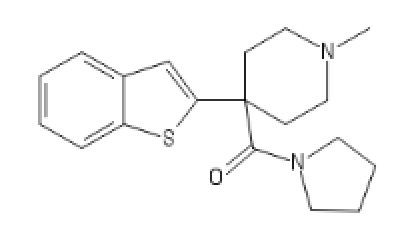
Should be opioïd, is Dopaminergic and Opioïd sigma active:
C2(CC(CC1=CC=CC=C1)N(CC2)[H])(C(=O)CC)C3=CC=CC=C3
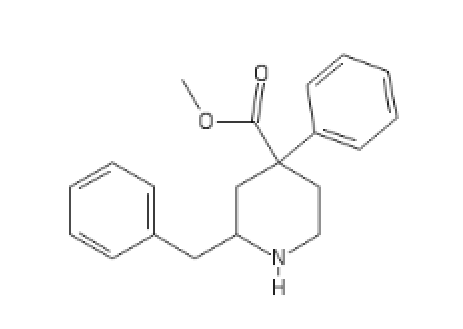
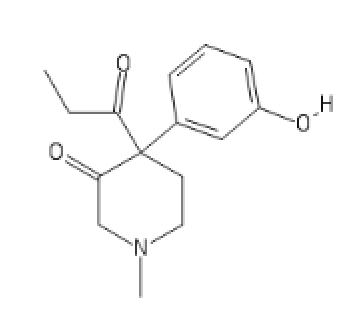
Ketobemidone analog:
C1(C(CN(CC1)C)=O)(C(=O)CC)C2=CC(=CC=C2)O[H]
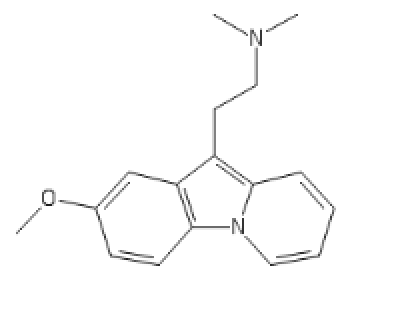
Chlorphenamine derived Antihistaminergic Psychedelics, or chillaxed tripping!
5-Meo one
C1=C(C=CC2=C1C(=C3C=CC=C[N]23)CCN(C)C)O
Chlorphenamine inspired
C1=CC(=CC2=C1C(=C3C=CC=C[N]23)CCN(C)C)Cl
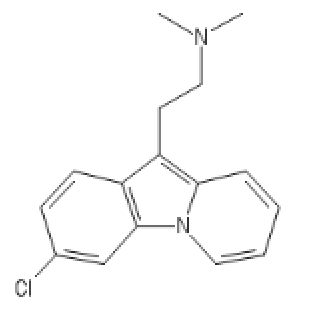
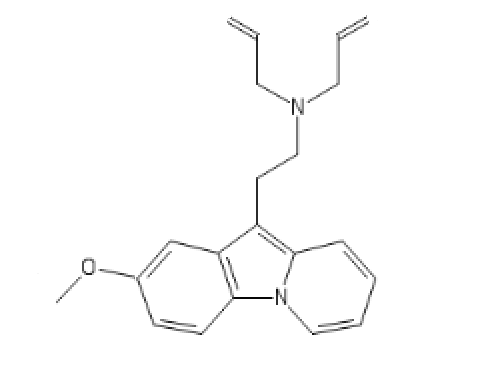
5-Meo DIPT one
C1=C(C=CC2=C1C(=C3C=CC=C[N]23)CCN(CC=C)CC=C)OC
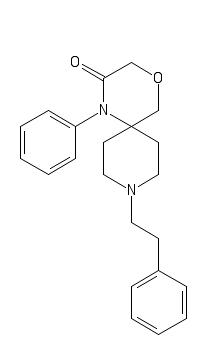
Now let's switch to killer opioïds, Fentanyl analogs and some others:
Cyclic R-30490
C1(=CC=CC=C1)CCC2CCC3(CC2)COCC(N3C4=CC=CC=C4)=O
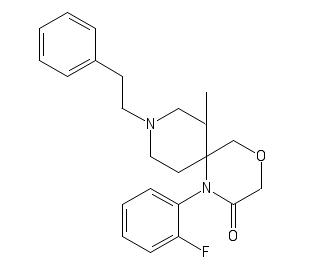
Its (probably) more potent analog, 2'-Fluoro-3-Methyl-Cyclic R-30490
C1(=CC=CC=C1)CCN2CC(C4(CC2)N(C3=C(C=CC=C3)F)C(COC4)=O)C
My FuranylFentanyl
C1(=CC=CC=C1)CCN2CCC(CC2)(C3=CC=CO3)N(C4=CC=CC=C4)C(CC)=O
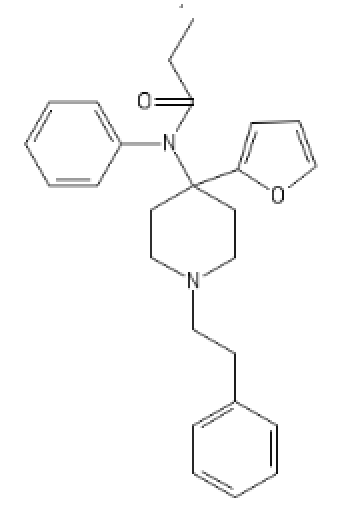
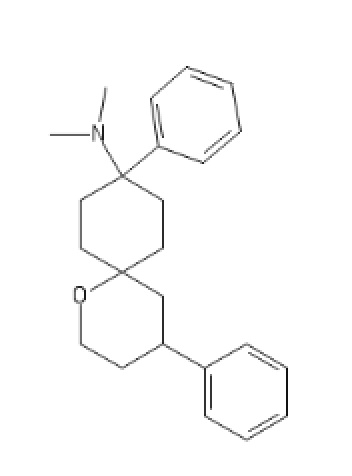
Non-Brominated Cyclic BDPC
C1=CC=C(C=C1)C2(CCC3(CC2)OCCC(C3)C4=CC=CC=C4)N(C)C
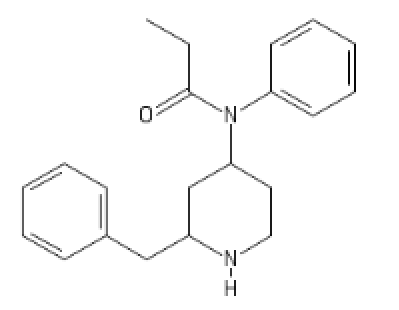
Fentanyl with the phenethyl displaced
C2(CC(CC1=CC=CC=C1)N(CC2)[H])N(C(CC)=O)C3=CC=CC=C3
And an already seen one, but it has its place here:
An outstanding one,both SNDRI and MOR (!)

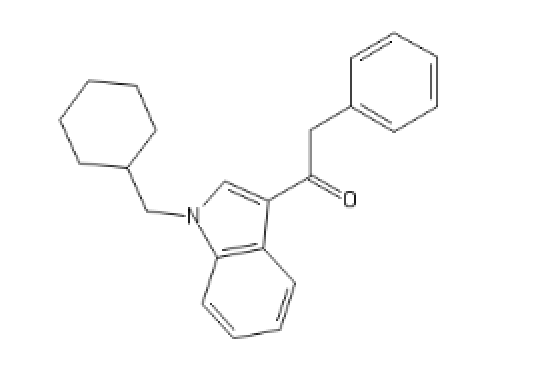
Some weird cannabinoïd, related to JWH-018
C1=CC=CC2=C1C(=C[N]2CC3CCCCC3)C(=O)CC4=CC=CC=C4
What do I call this:
C1=CC=CC2=C1C(=C[N]2CC3CCCCC3)C(=O)C5C4CCCCC4CCC5
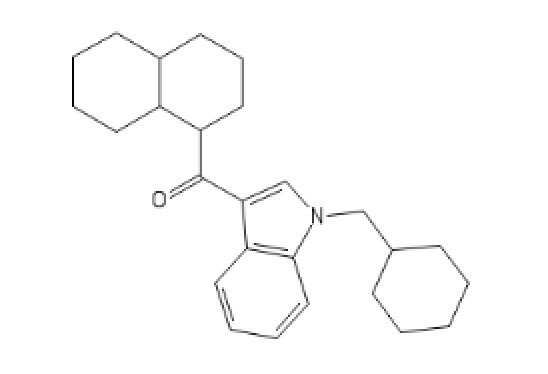
For more fun, go see ILTDPORM or Dresden's thread.
From here on the thread is dedicated to chems that aren't of my invention, mine are above ^.
Some of polymath's inventions:
N-PE-Nor-MEthadone
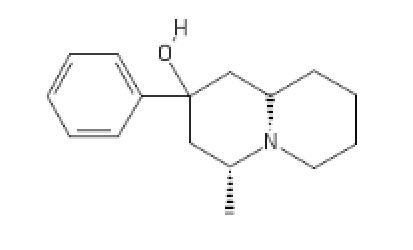
Polymath's Funny little beast, Fenmyrtin:
[C@H]1(CC(CC2CCCCN12)(C3=CC=CC=C3)O[H])C
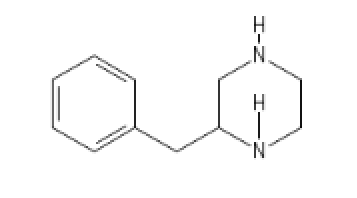
S.J.B.'s constitutional isomer of BZP
C1=CC=CC=C1CC2CN(CCN2[H])[H]
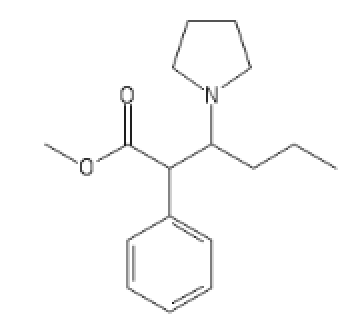
Dresden's Carbomethoxy PVP:
C1=CC=CC=C1C(C(CCC)N2CCCC2)C(=O)OC
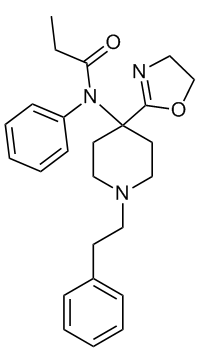
Sekio's Oxazolino Carfentanyl
C1(CCN(CC1)CCC2=CC=CC=C2)(C3=NCCO3)N(C4=CC=CC=C4)C(CC)=O
It'll take some time to finish this thread (if I ever do as I've litterally thousands of chemicals to sort out and run through, and test with the SAR machine), but I'll post what I think deserves to be posted.
We'll start with Ketamine and Ephenidine analogs I've invented, and switch to BTCP analogs which will permit us to switch to DRIs: (And from there it'll be pretty random, as I can't classify all my compounds)
Ketamine
PCP
Ephenidine
Seven ringed Ketamine,
CycloHeptylKetamine : C1(C(CCCCC1)=O)(C2=CC=CC=C2)N(C)[H]

Seven ringed Methoxetamine,
CycloHeptylMethoxetamine: C1C(C(CCCC1)(C2=CC=CC(=C2)OC)NCC)=O

Adding a benzene group to the cycloheptyl:
3-MeO-PCPy with a PhenylCycloHEptyl ring: C1CC(CCC2=C1C=CC=C2)(C3=CC=CC(=C3)OC)N4CCCC4

a little extra
(With very interesting SARs)
MorphoKetamine: ClC1=C(C=CC=C1)C1NCCOC1C1=CC=CC=C1

PCP with a N-PhenylPiperazine
PCPP: C1=CC=C(C=C1)N2CCN(CC2)C3(CCCCC3)C4=CC=CC=C4

BTCPP, BTCP with a phenylpiperazine
BTCPP: C1=CC=C(C=C1)N2CCN(CC2)C3(CCCCC3)C5=CC4=CC=CC=C4S5

Three analogs between Ephenidine and BTCP:
BTPhenEthylPiperidine : C(C(N1CCCCC1)C1=CC2=C(S1)C=CC=C2)C1=CC=CC=C1

BTPhenEthylPyrrolidine: C12=C(C=CC=C1)SC(=C2)C(CC3=CC=CC=C3)N4CCCC4

BTBKPhenEthylEthylamine: C12=C(C=CC=C1)SC(=C2)C(C(C3=CC=CC=C3)=O)N(CC)[H]

The morpholine analog of ketamine applied to BTCP:
MorphoBTCE: C1COC(C(N1)C1=CC2=C(S1)C=CC=C2)C1=CC=CC=C1

Mono-methylated alpha-phenylated tryptamine, a SNDRI that stands out:
N-Methyl-Alpha-Phenyl-Tryptamine: C1=CC=CC2=C1C(=C[N]2)CC(C3=CC=CC=C3)NC

Its N-Pyrrolidino analog
5-MeO-AMT
C1(=CC=C2C(=C1)C(=C[N]2)CC(N([H])[H])C)O

Cocaïne analogs: probably my best inventions
I Don't know what to call these, but they're wonderously promising
COC(=O)C(CC1=CC=CC=C1)C1CCCN1

The N-Methyl, even more promising
C1(=CC=CC=C1)CC(C(=O)OC)C2CCCN2C

The local anaesthetic version, it's got high HERG activity:
C1=CC=CC=C1C(OCC(C(OC)=O)C2CCCN2[H])=O

The N-Methyl version, with even more HERG activity, which makes it cardiotoxic
COC(=O)C(COC(=O)C1=CC=CC=C1)C1CCCN1C

An outstanding one,both SNDRI and MOR (!)

N'-N-Methyl version
2-FA Light
C1(=CC=CC=C1OC2CCN(CC2)[H])F

4-MethylMethylphenidate N-pyrrolidine analog
C1=CC=CC=C1C(C(N2CCCC2)=O)C3CCCCN3[H]

PVP Methylphenidate: Surprisingly good
C1=CC=CC=C1C(C2CCCC3CCCN23)C(OC)=O

N-pyrrolidino Version
3-MeO-PhenEtrazine
C1=C(C=CC=C1C2C(CC)N(CCO2)[H])OC

CyclopentylEthylCathinone
CCNC(C1CCCC1)C(=O)C1=CC=CC=C1

Two Ketobemidone variants, go guess how I guessed
BTKETO1
C1=CC=C2C(=C1)C=C(S2)C3(CCN(CC3)C)CN4CCCC4

BTKETO2
C1=CC=C2C(=C1)C=C(S2)C3(CCN(CC3)C)C(N4CCCC4)=O

Should be opioïd, is Dopaminergic and Opioïd sigma active:
C2(CC(CC1=CC=CC=C1)N(CC2)[H])(C(=O)CC)C3=CC=CC=C3

Ketobemidone analog:
C1(C(CN(CC1)C)=O)(C(=O)CC)C2=CC(=CC=C2)O[H]

Chlorphenamine derived Antihistaminergic Psychedelics, or chillaxed tripping!
5-Meo one
C1=C(C=CC2=C1C(=C3C=CC=C[N]23)CCN(C)C)O

Chlorphenamine inspired
C1=CC(=CC2=C1C(=C3C=CC=C[N]23)CCN(C)C)Cl

5-Meo DIPT one
C1=C(C=CC2=C1C(=C3C=CC=C[N]23)CCN(CC=C)CC=C)OC

Now let's switch to killer opioïds, Fentanyl analogs and some others:
Cyclic R-30490
C1(=CC=CC=C1)CCC2CCC3(CC2)COCC(N3C4=CC=CC=C4)=O

Its (probably) more potent analog, 2'-Fluoro-3-Methyl-Cyclic R-30490
C1(=CC=CC=C1)CCN2CC(C4(CC2)N(C3=C(C=CC=C3)F)C(COC4)=O)C

My FuranylFentanyl
C1(=CC=CC=C1)CCN2CCC(CC2)(C3=CC=CO3)N(C4=CC=CC=C4)C(CC)=O

Non-Brominated Cyclic BDPC
C1=CC=C(C=C1)C2(CCC3(CC2)OCCC(C3)C4=CC=CC=C4)N(C)C

Fentanyl with the phenethyl displaced
C2(CC(CC1=CC=CC=C1)N(CC2)[H])N(C(CC)=O)C3=CC=CC=C3

And an already seen one, but it has its place here:
An outstanding one,both SNDRI and MOR (!)

Some weird cannabinoïd, related to JWH-018
C1=CC=CC2=C1C(=C[N]2CC3CCCCC3)C(=O)CC4=CC=CC=C4

What do I call this:
C1=CC=CC2=C1C(=C[N]2CC3CCCCC3)C(=O)C5C4CCCCC4CCC5

For more fun, go see ILTDPORM or Dresden's thread.
From here on the thread is dedicated to chems that aren't of my invention, mine are above ^.
Some of polymath's inventions:
N-PE-Nor-MEthadone
Polymath's Funny little beast, Fenmyrtin:
[C@H]1(CC(CC2CCCCN12)(C3=CC=CC=C3)O[H])C

S.J.B.'s constitutional isomer of BZP
C1=CC=CC=C1CC2CN(CCN2[H])[H]

Dresden's Carbomethoxy PVP:
C1=CC=CC=C1C(C(CCC)N2CCCC2)C(=O)OC

Sekio's Oxazolino Carfentanyl
C1(CCN(CC1)CCC2=CC=CC=C2)(C3=NCCO3)N(C4=CC=CC=C4)C(CC)=O

Last edited:
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
Here's my N-phenethyl-nortramadol submitted there...
 www.swisstargetprediction.ch
www.swisstargetprediction.ch
and here is the N-phenethyl-nor-eseroline with the 2-phenylethyl put on the nitrogen that is a longer distance away from the aromatic ring...
 www.swisstargetprediction.ch
www.swisstargetprediction.ch
SwissTargetPrediction
and here is the N-phenethyl-nor-eseroline with the 2-phenylethyl put on the nitrogen that is a longer distance away from the aromatic ring...
SwissTargetPrediction
- Status
- Not open for further replies.