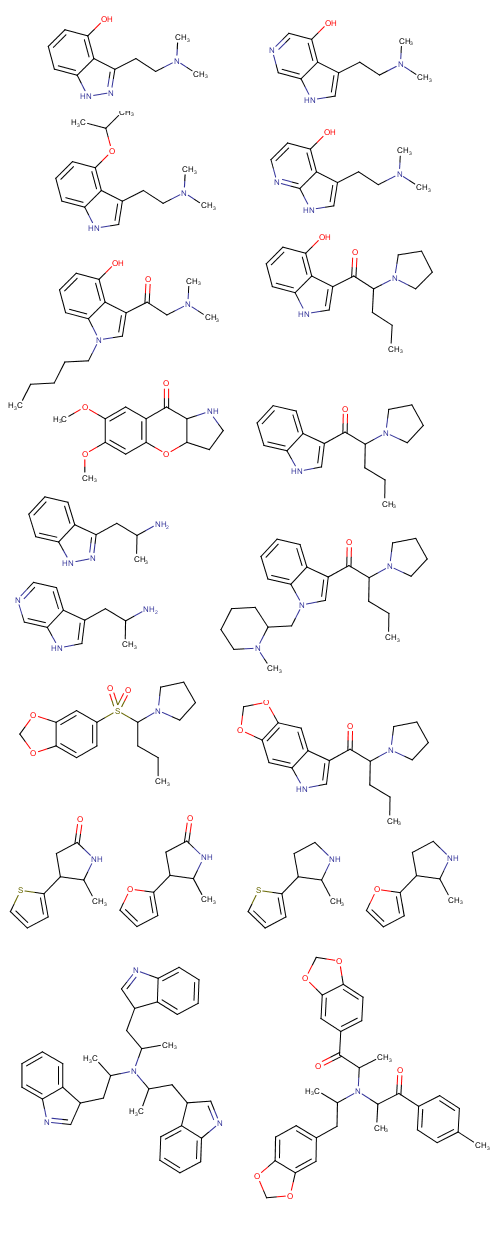
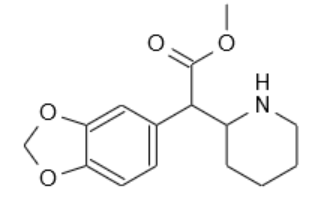
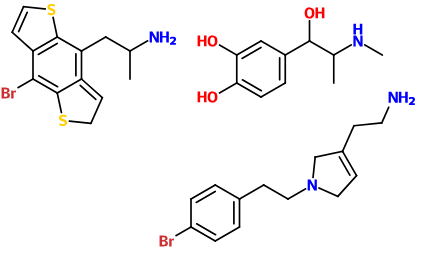
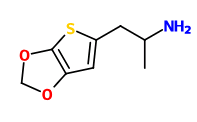
Soulfake
Bluelighter
- Joined
- Aug 12, 2010
- Messages
- 160
@hodor
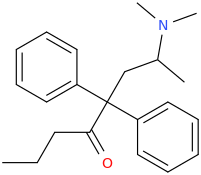
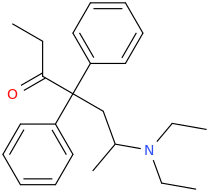
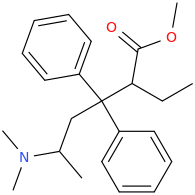
Thx, I got that wrong. Somehow I had in mind that theanine was an ethyl-ester but it's an ethylamide analogue of glutamine. Do esters and amides behave similar in some ways? As far as I have seen/read both esters and amides often hydrolize in the body, depending on the molecule structure more or less fast while I guess most esters do hydrolize quicker than amides (I'm just a layman in chemistry and don't have real knowledge about basic and complex stuff, just a few pieces of information I read over the last 10 years about rc's and substances in general.)
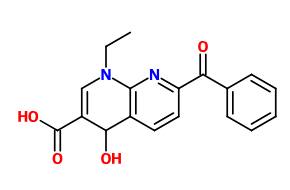
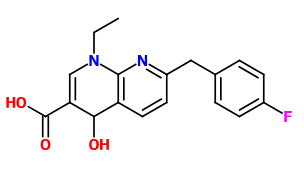
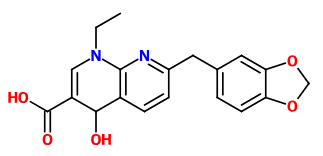
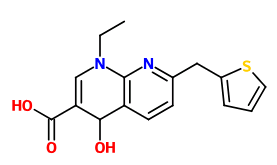
I read a research paper where esters of GABA with inositol where mentioned as a method of delivering it more efficient into the CNS (I can't find it anymore but these ones also describe it: https://www.researchgate.net/public...de_Analgesia_for_Some_Central_Pain_Conditions https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2918363/
I don't think it's recreational potential is really lower, maybe different but at least where I live it's very popular, most people I know who have tried pregabalin in higher doses (300-500mg) rate it much higher than benzos. I only have tried once 150mg+150mg a few hours later and it was really strong, when I stood up I always stumbled around and the muscle relaxation was really heavy, a bit like alcohol just without the mind feeling of it, much more clear and bright with a heavy euphoria, I was in a social setting with friends and although I also find some benzos euphoric this was way stronger, I guess to get such a strong muscle relaxation you would need so many benzos that it'll cause blackouts.
But I also guess that it would be hard to make/find really good gabapentinoids as "research chemicals", but that's why I thought that some kind of pro-drug for known ones like pregabalin would make most sense. I believe that there could be some simple analogues/pro-drugs that would be as cheap as for example phenibut, GABOB, picamilon etc. Pregabalin is just GABA with that ethyl-isopropyl unit or like gabapentin with an cyclohexane, GABOB with an OH-unit etc. I also think the chance for severe toxicity of simple amino acid analogues is a bit lower than for example all those cannabinoid and cathinone analogues, or not? I know that some strong poisons have amino acids build into their structure but quite differently than those mentioned structure modifications.
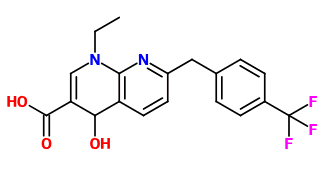
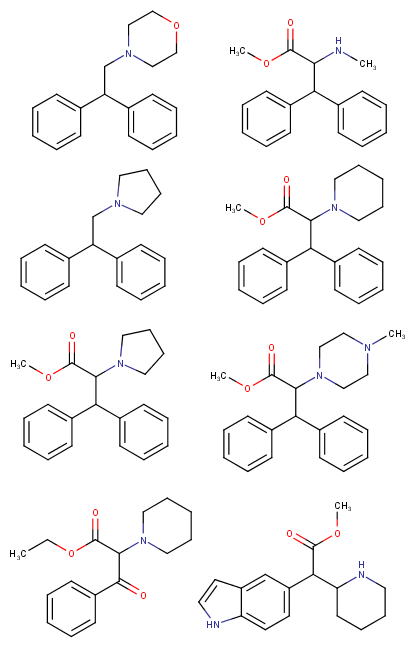
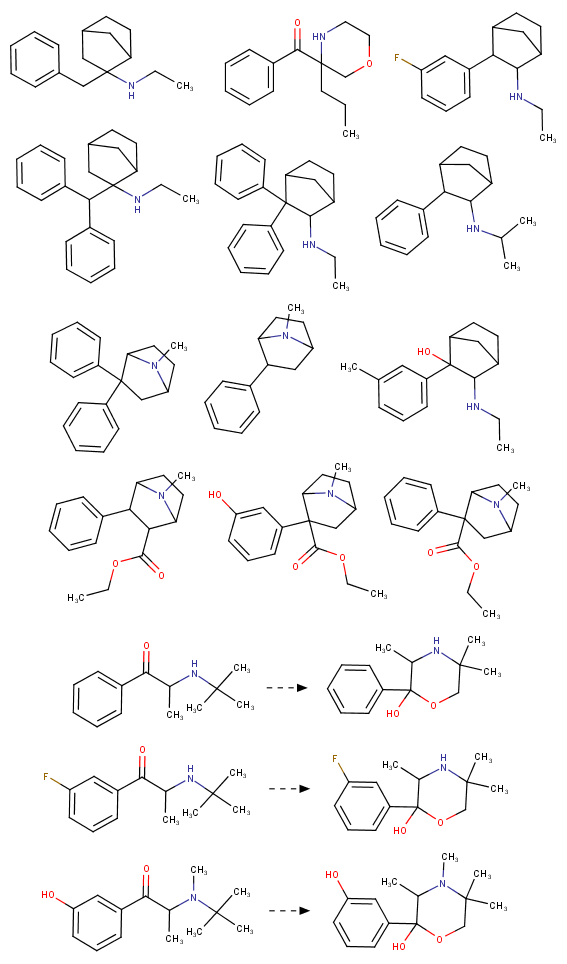
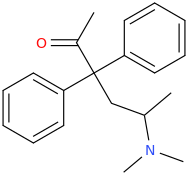
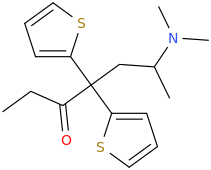
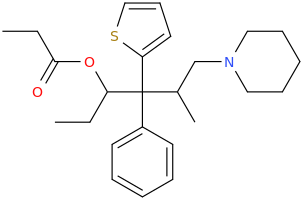
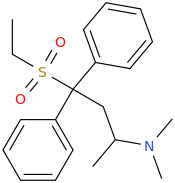
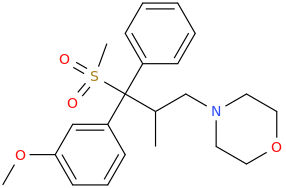
I know about methylmorphenate, I guess that making a para-fluoro or (di)chloro-substitution could increase it's effects? But I plan to get a small sample just for the collection as I really like it's structure and morpholines in general. Maybe it's just an imaginary delusion to think morpholine and norbornane stimulants are much smoother and enjoyable but I really liked camfetamine and 3fpm so I'm a bit framed about this.
There is no such thing as an "N-ethyl ester". Condensation of an acid with an amine forms an amide.
Thx, I got that wrong. Somehow I had in mind that theanine was an ethyl-ester but it's an ethylamide analogue of glutamine. Do esters and amides behave similar in some ways? As far as I have seen/read both esters and amides often hydrolize in the body, depending on the molecule structure more or less fast while I guess most esters do hydrolize quicker than amides (I'm just a layman in chemistry and don't have real knowledge about basic and complex stuff, just a few pieces of information I read over the last 10 years about rc's and substances in general.)
Why inositol of all things, though? Inositol has a greater molar mass than pregabalin itself, and it is hydrophilic as hell, so its bioavailability might actually be worse.
I read a research paper where esters of GABA with inositol where mentioned as a method of delivering it more efficient into the CNS (I can't find it anymore but these ones also describe it: https://www.researchgate.net/public...de_Analgesia_for_Some_Central_Pain_Conditions https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2918363/
Compared to benzodiazepines, pregabalin's recreational potential is far lower. Also, one of the problems with gabapentinoids is that their structure-activity-relationship is still not all that well-understood. Gabapentinoids apparently need to be actively transported into the brain via special amino acid carrier proteins. For years, the search for a successor to pregabalin has been slowed down by the fact that compounds that seemed much more potent in the in-vitro binding assays showed little if any increase in in-vivo potency, as their structural modifications would also decrease affinity for the aforementioned amino acid carriers, no longer justifying the significantly more complex synthesis.
I don't think it's recreational potential is really lower, maybe different but at least where I live it's very popular, most people I know who have tried pregabalin in higher doses (300-500mg) rate it much higher than benzos. I only have tried once 150mg+150mg a few hours later and it was really strong, when I stood up I always stumbled around and the muscle relaxation was really heavy, a bit like alcohol just without the mind feeling of it, much more clear and bright with a heavy euphoria, I was in a social setting with friends and although I also find some benzos euphoric this was way stronger, I guess to get such a strong muscle relaxation you would need so many benzos that it'll cause blackouts.
But I also guess that it would be hard to make/find really good gabapentinoids as "research chemicals", but that's why I thought that some kind of pro-drug for known ones like pregabalin would make most sense. I believe that there could be some simple analogues/pro-drugs that would be as cheap as for example phenibut, GABOB, picamilon etc. Pregabalin is just GABA with that ethyl-isopropyl unit or like gabapentin with an cyclohexane, GABOB with an OH-unit etc. I also think the chance for severe toxicity of simple amino acid analogues is a bit lower than for example all those cannabinoid and cathinone analogues, or not? I know that some strong poisons have amino acids build into their structure but quite differently than those mentioned structure modifications.
I know about methylmorphenate, I guess that making a para-fluoro or (di)chloro-substitution could increase it's effects? But I plan to get a small sample just for the collection as I really like it's structure and morpholines in general. Maybe it's just an imaginary delusion to think morpholine and norbornane stimulants are much smoother and enjoyable but I really liked camfetamine and 3fpm so I'm a bit framed about this.
Last edited: