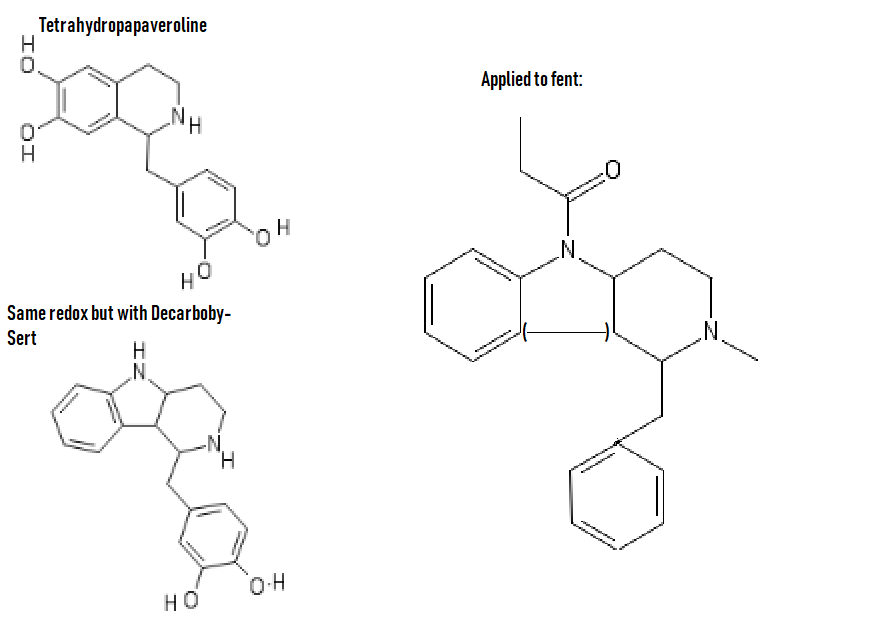
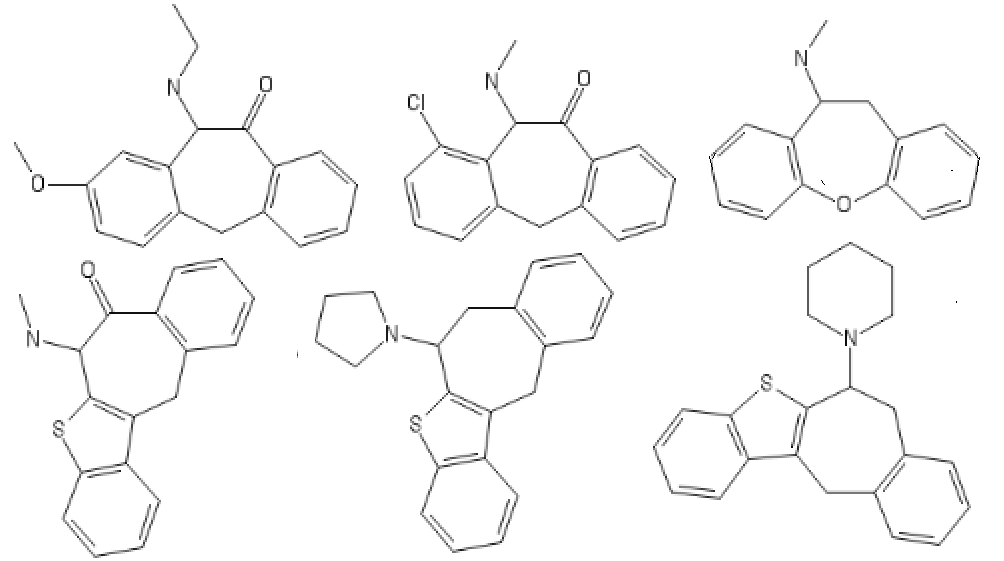
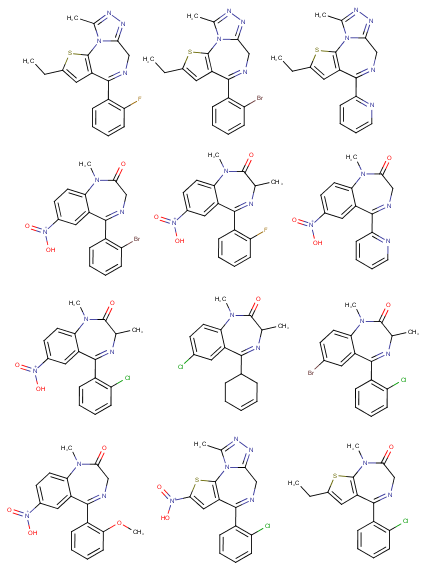
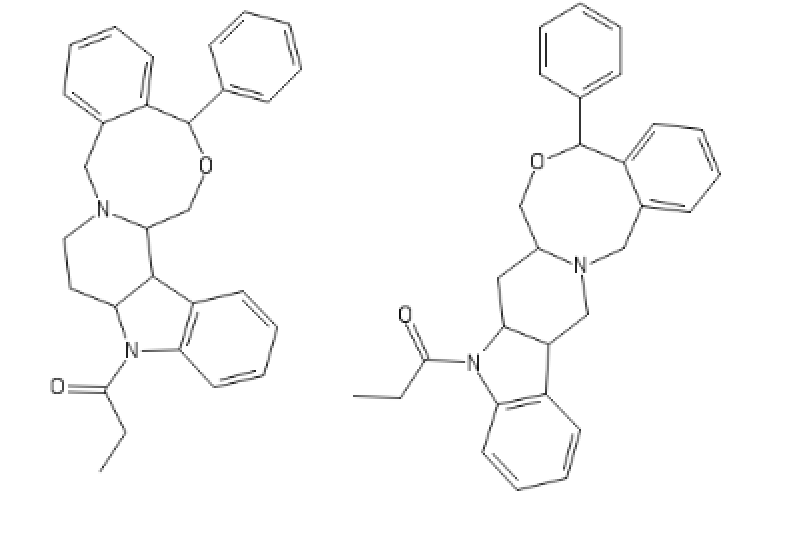
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
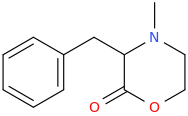
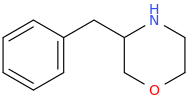
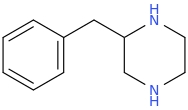
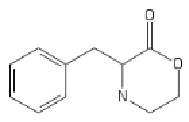
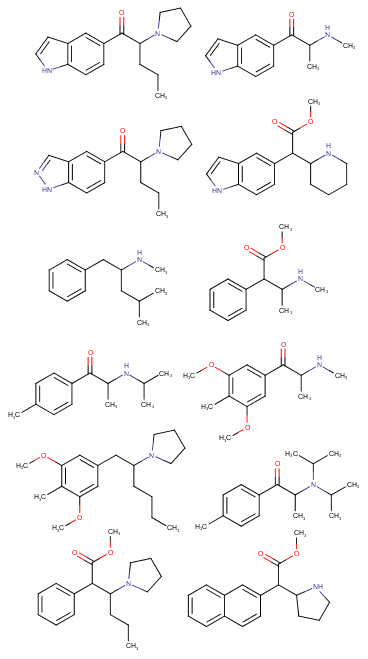
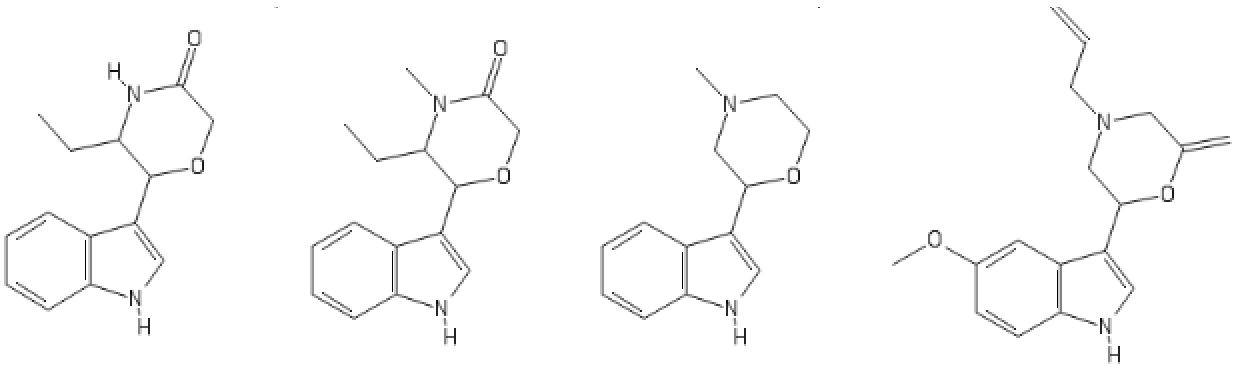
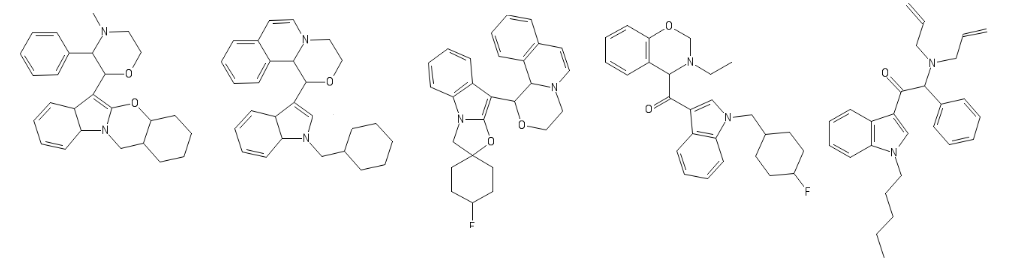
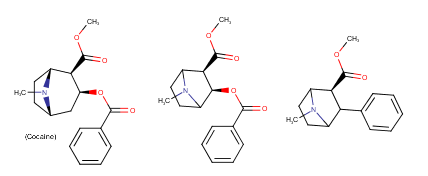
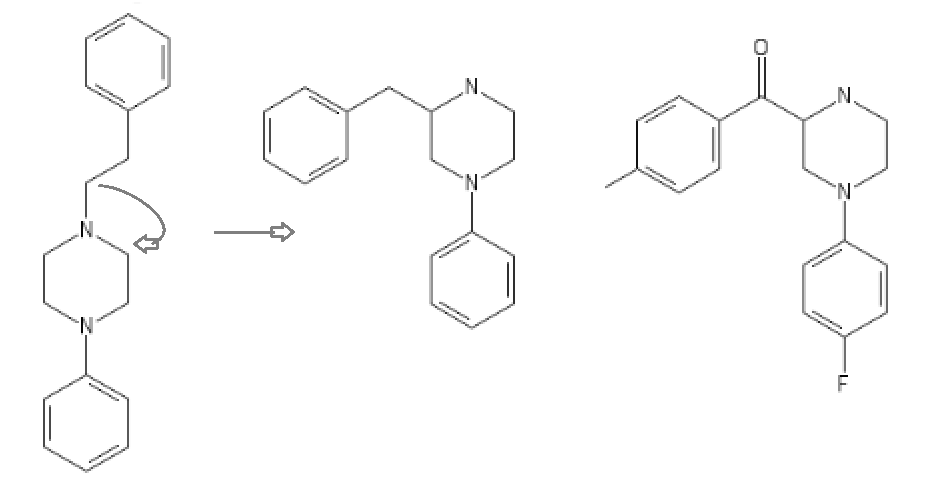
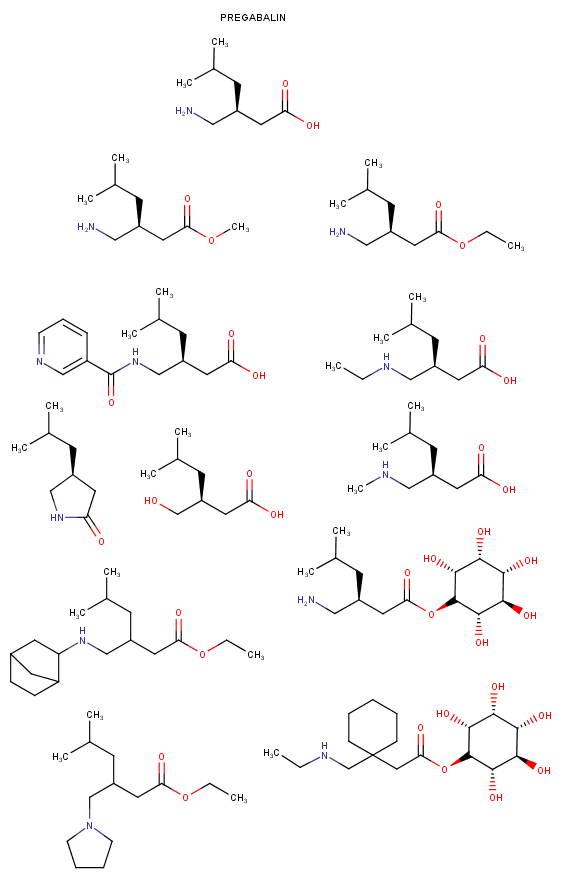
Yeah, I meant the compound with a 6-membered ring. I thought that could potentially bind to monoamine transporters. Another possibility is the cyclic amide from 1-amino-2-bromoethane and phenylalanine, but then there would be more polymeric byproducts formed.