polymath
Bluelight Crew
^ It must have been a bit of work drawing all of those and making predictions...
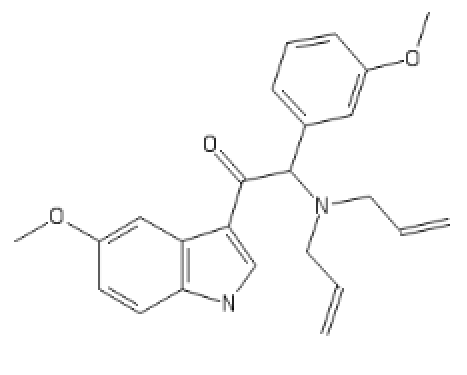
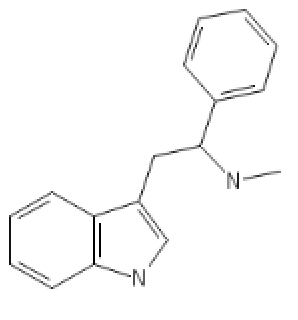
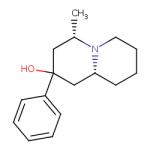
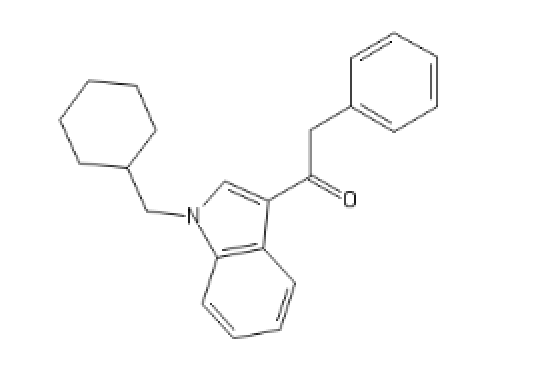
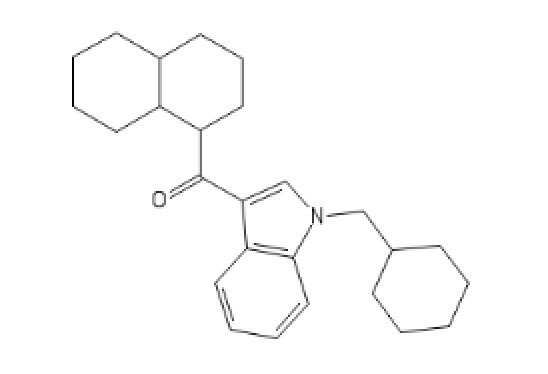
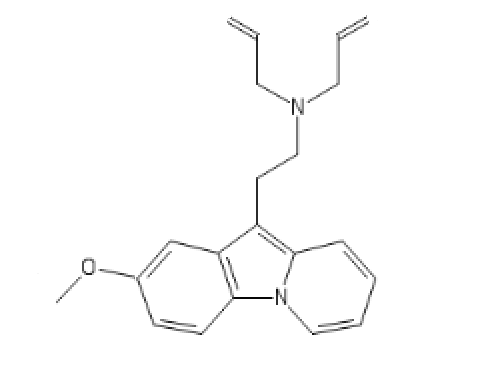
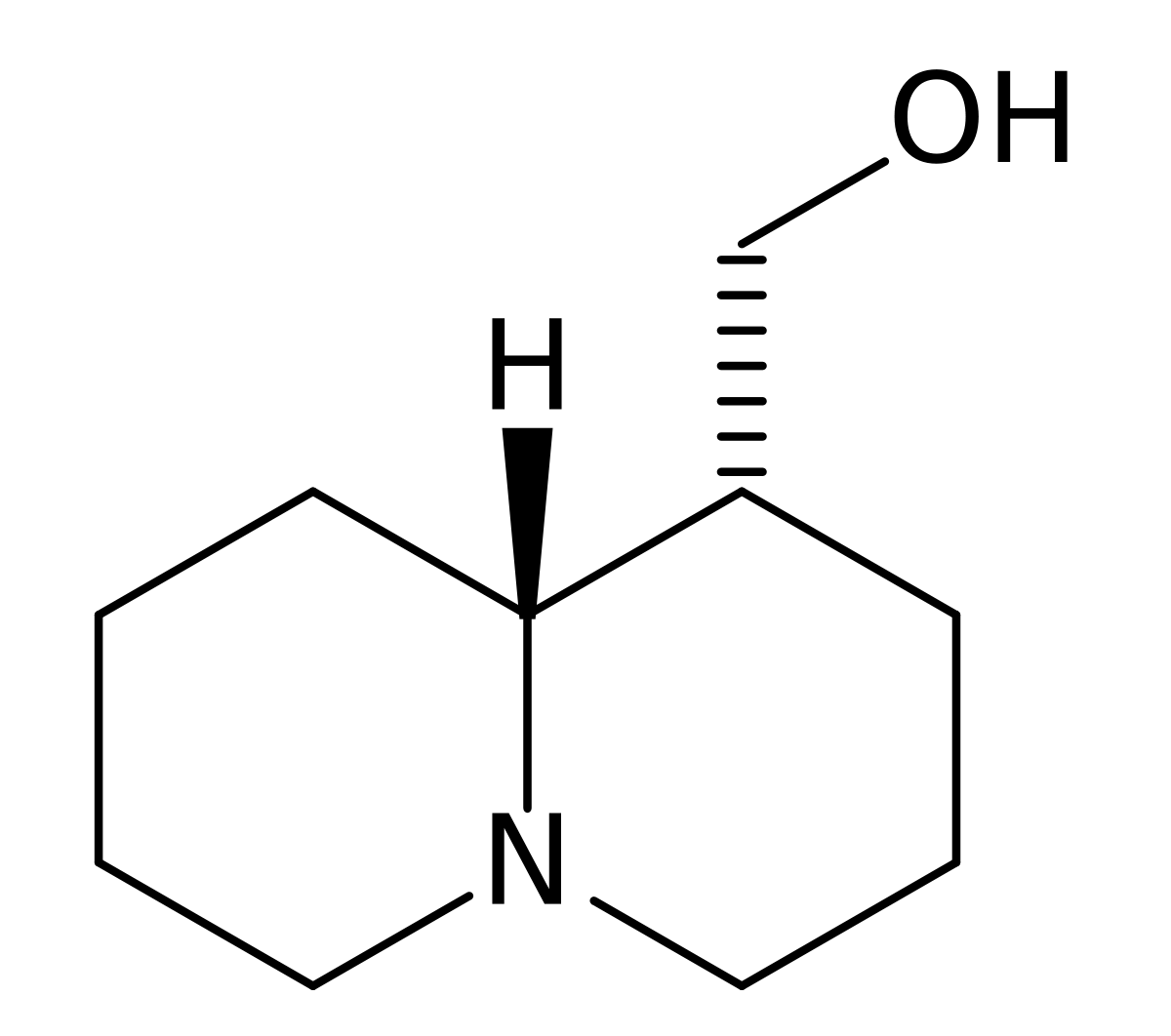
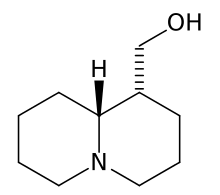
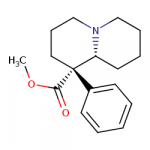
I tried to put a non-amine version of methylphenidate, with a sulfur atom in place of nitrogen, in the Swiss target prediction app as an input (the oxygen version is known to be a likely DAT ligand). Didn't get a very high likelihood of binding to dopamine transporter. But when checking the list of similar structures it identified that with, I saw this crazy biphenyl compound that is known to inhibit both dopamine and serotonin uptake:
 www.ebi.ac.uk
www.ebi.ac.uk
probably the strangest stimulant candidate I've seen...
I tried to put a non-amine version of methylphenidate, with a sulfur atom in place of nitrogen, in the Swiss target prediction app as an input (the oxygen version is known to be a likely DAT ligand). Didn't get a very high likelihood of binding to dopamine transporter. But when checking the list of similar structures it identified that with, I saw this crazy biphenyl compound that is known to inhibit both dopamine and serotonin uptake:
Compound: CHEMBL101986 - ChEMBL
Report card for Compound CHEMBL101986. C14H11ClO2, Small molecule.
probably the strangest stimulant candidate I've seen...