Xorkoth
Bluelight Crew
Okay so I have a number of very rare drugs and a few I wanted to confirm. A friend of mine works with an organization that just developed a device that can read a tiny sample of a drug and print out the infrared mass spectra results, he claims it's a revolution is simplicity of identifying drugs. However basically none of the drugs I sent him have a reference stored into the machine so it just came up with guesses it considered close, but he told me not to worry, that it just didn't know, but he gave me the readouts as .png files. I'm hoping one of you intelligent souls can read this stuff and give me any insight. The three I care the most about are DOF, DOiP, and DOPr. The DOF in particular is, as far as I know, the only DOF anywhere. I wrote the first full trip report on it and, well, any other TRs forthcoming are likely to be from the same batch. There are also a couple of other drugs that are new to the scene that I don't know of anyone testing before. So, obviously, I'm hoping I can verify their identity.
What I'm going to do is post each in an NSFW with what it's supposed to be, what the machine said it was, and the spectra image. Thanks so much for any help. Keep in mind this is a brand new device so for all I know it might be inaccurate. It did get one of them correct and it was the one he said they had in their database already (escaline). This was free, I would have preferred to send off to a lab for GC/MS but being in the US, that's stupidly expensive. If anyone is able to help, you'd be doing a favor to the community as a whole because some of these things are extremely rare or just released for the first time from a single source.
Also my friend said that if, for example, their reference was for the HCl salt of a drug, and I have the fumarate or some other salt, it would come up wrong because of a different molecular weight.
Supposed to be DOF, the machine said 2C-E HCL - it is most absolutely clearly not 2C-E
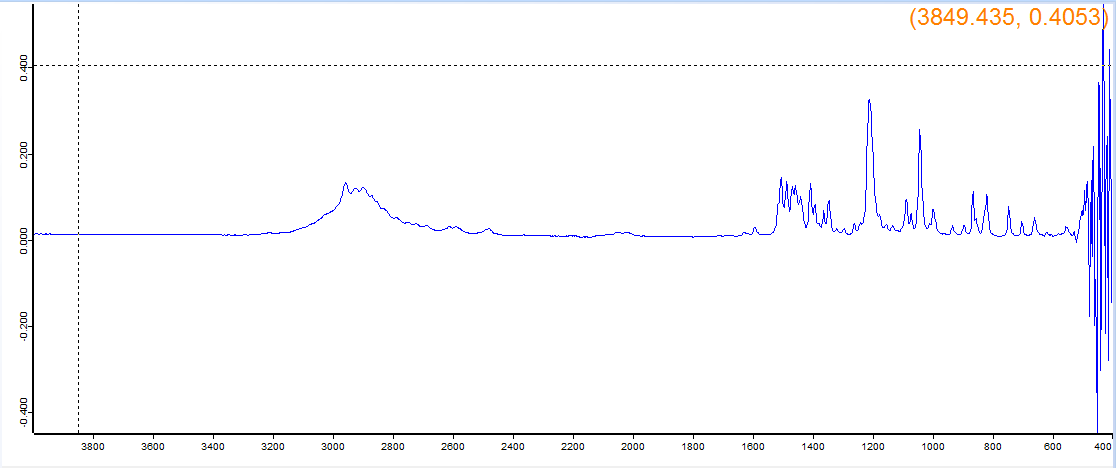
Supposed to be BOD, the machine said 5,6-Methylenedioxy-2-aminoindane
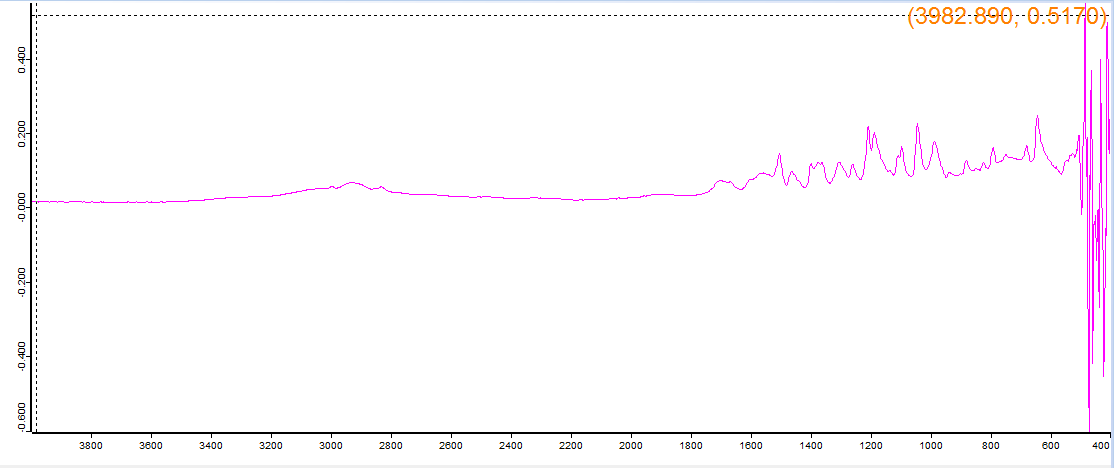
Supposed to be proscaline, the machine said mescaline sulfate dihydrate
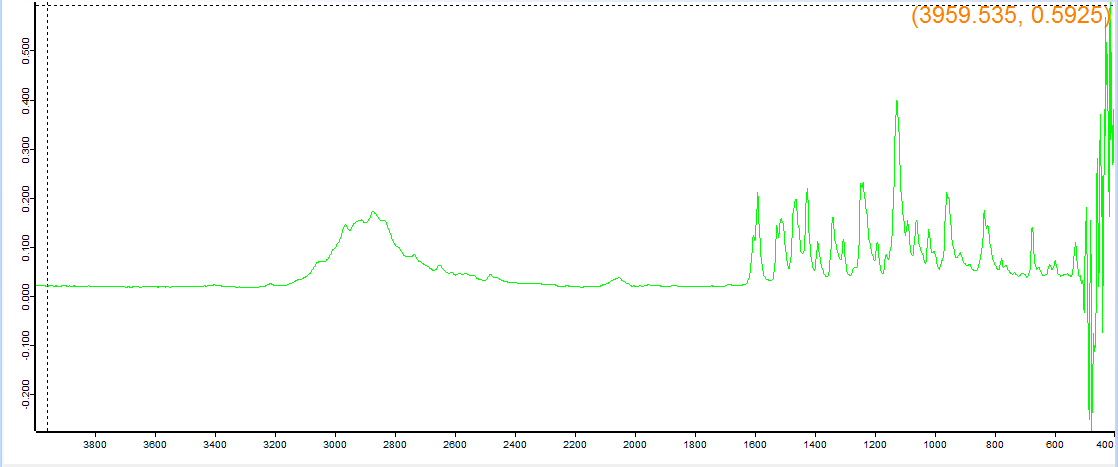
For reference, I had this one lab tested and it was actually good so if the readout with this one looks funny I think it means the machine doesn't work right.
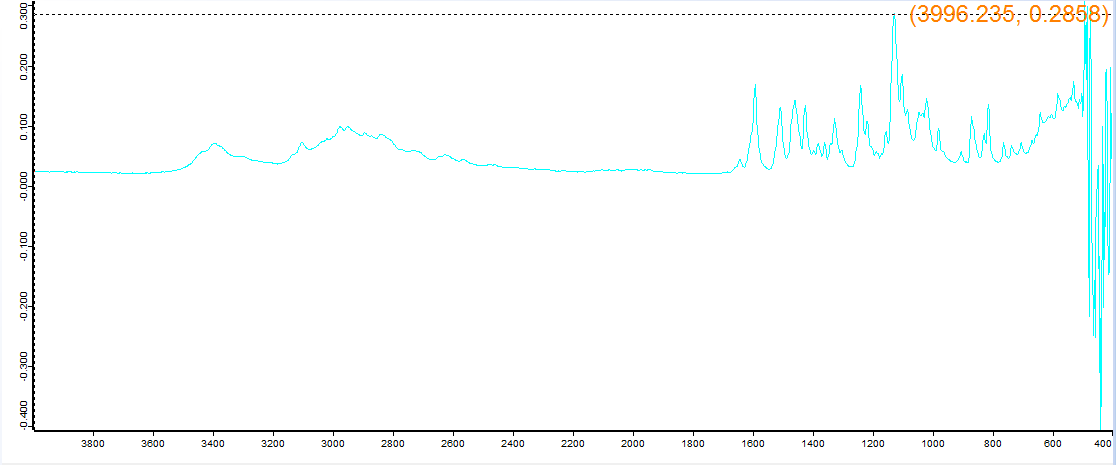
Supposed to be escaline, the machine said escaline HCl so it matched, but would like to confirm
Supposed to be 4C-D (ARIADNE from PIHKAL), the machine said 2C-E HCl (same as it did for DOF). This is also EXTREMELY obviously not 2C-E.
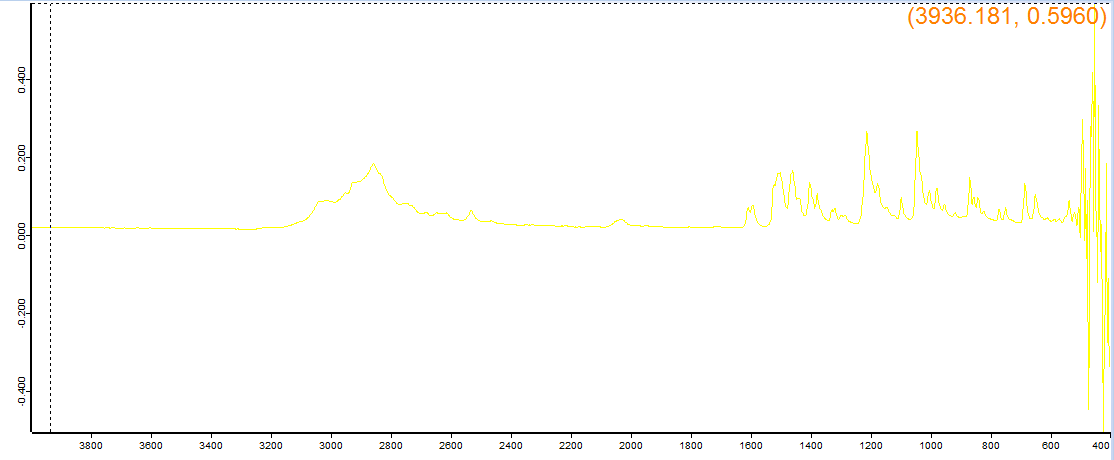
Supposed to be 3C-P, the machine said 4-Chloropentedrone HCl. This is obviously not that as it matches duration, potency and style of effect of the 3C-P reports I've read, as opposed to some shitty cathinone.
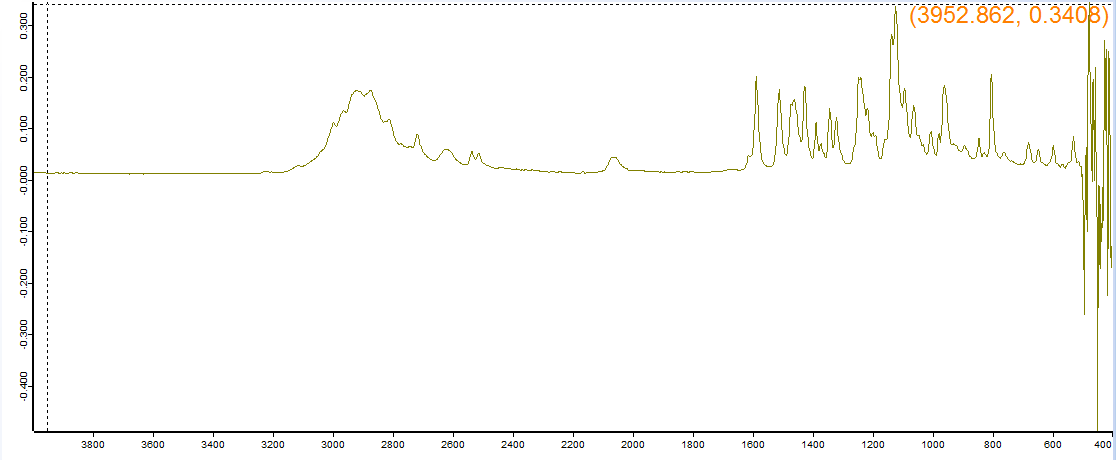
Supposed to be 3C-E, the machine said 4-Fluoroamphetamine HCl. Suffice to say, it is not 4-FA by any stretch of the imagination
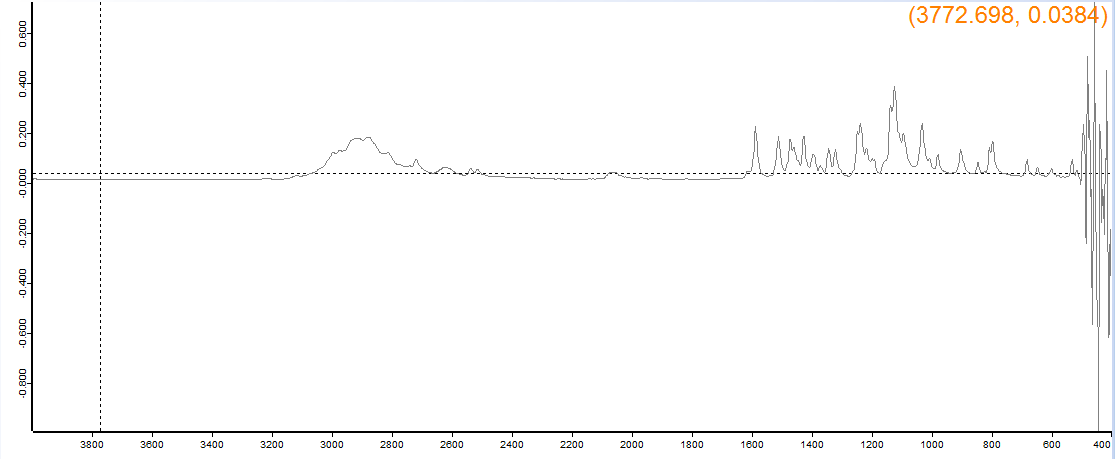
Supposed to be 5-MeO-MiPT fumarate, and the machine says it's 5-MeO-DPT HCl, which it couldn't be as the effects match 5-MeO-MiPT and 5-MeO-DPT is described as physically worrisome. But probably due to different molecular weight of fumarate salts.
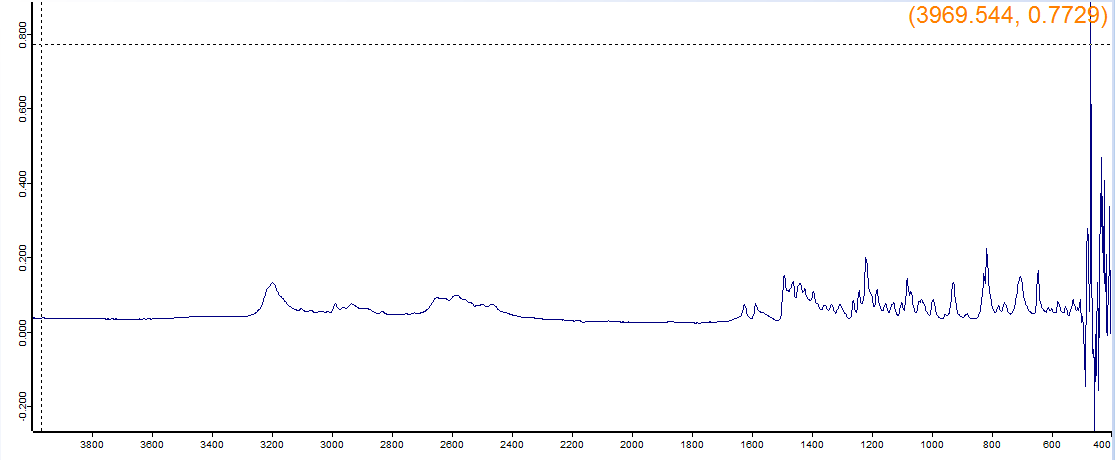
Thanks again for any help or feedback. I really have to get some of these positively IDd and I'm hoping it's possible to do from the information in this post, but if not, let me know and I'll send to a lab.
What I'm going to do is post each in an NSFW with what it's supposed to be, what the machine said it was, and the spectra image. Thanks so much for any help. Keep in mind this is a brand new device so for all I know it might be inaccurate. It did get one of them correct and it was the one he said they had in their database already (escaline). This was free, I would have preferred to send off to a lab for GC/MS but being in the US, that's stupidly expensive. If anyone is able to help, you'd be doing a favor to the community as a whole because some of these things are extremely rare or just released for the first time from a single source.
Also my friend said that if, for example, their reference was for the HCl salt of a drug, and I have the fumarate or some other salt, it would come up wrong because of a different molecular weight.
NSFW:
Supposed to be DOF, the machine said 2C-E HCL - it is most absolutely clearly not 2C-E

NSFW:
Supposed to be BOD, the machine said 5,6-Methylenedioxy-2-aminoindane

NSFW:
Supposed to be proscaline, the machine said mescaline sulfate dihydrate

For reference, I had this one lab tested and it was actually good so if the readout with this one looks funny I think it means the machine doesn't work right.
NSFW:
Supposed to be escaline, the machine said escaline HCl so it matched, but would like to confirm

NSFW:
Supposed to be 4C-D (ARIADNE from PIHKAL), the machine said 2C-E HCl (same as it did for DOF). This is also EXTREMELY obviously not 2C-E.

NSFW:
Supposed to be 3C-P, the machine said 4-Chloropentedrone HCl. This is obviously not that as it matches duration, potency and style of effect of the 3C-P reports I've read, as opposed to some shitty cathinone.

NSFW:
Supposed to be 3C-E, the machine said 4-Fluoroamphetamine HCl. Suffice to say, it is not 4-FA by any stretch of the imagination

NSFW:
Supposed to be 5-MeO-MiPT fumarate, and the machine says it's 5-MeO-DPT HCl, which it couldn't be as the effects match 5-MeO-MiPT and 5-MeO-DPT is described as physically worrisome. But probably due to different molecular weight of fumarate salts.

Thanks again for any help or feedback. I really have to get some of these positively IDd and I'm hoping it's possible to do from the information in this post, but if not, let me know and I'll send to a lab.



