tregar
Bluelighter
Update 2.17.2023:
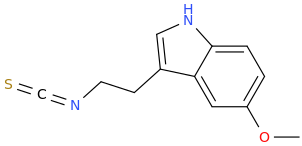
Sublingual Ayahuasca, my psychedelic of choice: https://www.shroomery.org/forums/showflat.php/Number/28189371
hxxps://www.shroomery.org/forums/showflat.php/Number/28189371 (change hxxp to http to link)
I've actually decided to save my bridgesii only for when I go outdoors to the waterpark with my family or in nature at the river. I always get a mild hangover from cactus that makes me extra tired, and I end up sleeping too much of the next day away, and my beautiful, funny and smart wife gets aggravated with me cause I can't stay up to watch movies.
cause I can't stay up to watch movies.
I actually went back to taking 200mg tetrahydroharmine + 200mg harmine orally at one time in a capsule, then 1 hour later when I feel it working, I place the HPBCD DMT under my tongue and wait for it dissolve which happens 100% in 15 minutes. It is no different from high dose cactus in effects for 1.5 hour with an incredible afterglow.
Sublingual Ayahuasca directions:
1) Take 200mg harmine freebase and 200mg pure THH freebase orally in a capsule
How to make pure THH or tetrahydroharmine here, post #13: https://mycotopia.net/topic/111610-hpbcd-dmt-sublingually-active-under-tongue/
2) 1 hour later: Place 120mg DMT on a spoon
cover with x 7 or 840mg Hydroxy propyl beta cyclodextrin or HPBCD also use the 2-Hpbcd, same in effect. This keeps it at a 1:1 molar ratio, as HPBCD (1300 g/mol, is x7 bigger in mol/g weight by dmt (188 g/mol).
3) add 10 drops of hot water from a microwaved coffee mug
4) mix and scrape it all back and forth for 1 to 2 minutes on the spoon using the end of another spoon, the complex is formed with absorption into the sublingual mucosa x 4 normal or 400% increase studies show.
5) Place bottom side of tongue onto spoon, the HPBCD DMT complex will all adhere, as HPBCD forms sticky complexes like sugar. Now hold under your tongue for 15 minutes, if at any time you feel too much saliva form, simply tilt head forward and spit out the extra saliva into a cup, but always keep tongue pressed down.
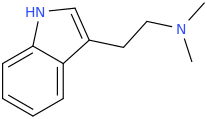
How this works: the oral harmine you took works in the brain to prevent the "zapping of dmt" by mitochondria located within the cells of the brain according to Dr. Mckenna, mao is reduced in the brain for several hours as harmine has a half life of around 1 to 3 hours. So once the dmt is absorbed sublingually, the harmine working in the brain prevents it from breaking down, and the THH doubles it's half life. The THH is critical as it is a major alkaloid of Caapi, a feminine teaching spirit who causes visions all on her own, she is the guide and teacher for the experience. She also blocks serotonin which causes mild stimulation, dmt does not block serotonin.
There is a mild sting but it is so worth the wait. The HPBCD being such a large molecular weight is not absorbed by the mucosa but does facilitate the transfer of the DMT into the bloodstream as the potent freebase by opening up of "tight junctions" as research into HPBCD shows. The HPBCD ends up in the saliva you spit out after 15 minutes. The mucosa under tongue is also only 100 to 200 micrograms thick, it is rich for this activity and many times more potent than normal oral absorption according to Dr. Narang.
When you mix it on the spoon, use your muscles to scrape, mix and knead it all together. This is how scientist prepare these complexes, by kneading.
1.5 hour incredible experience +5 shulgin in intensity and euphoria and beauty....beautiful geometrics overlaid all surfaces, music beyond heavenly, just incredible music enhancement...actresses on tv look like goddesses.
The colors omg are just neon like and out of this world, so beautiful, the shimmering of everything is beyond belief. The beauty with open eyes is divine and infinite. The interconnection of all things is seen, and very deep spiritual insights and healing. I walked out in nature and lost myself in the beauty.
None of the negatives of oral Ayahuasca, as oral DMT makes my very nauseated and uncomfortable, this is all positives...very euphoric and straight to bloodstream as the potent freebase.
Way better than LSD.....and just as good as high dose cactus tea. I had an experience tonight that blew my mind, this is my method to use every weekend for the rest of my life, Sublingual Ayahuasca is a great way to stay psychedelic for the rest of your life. I re-dosed more HPBCD DMT at the 1.5 hour point, and the fun continued for another 1.5 hour, as the oral harmine has a half life of from 1 to 3 hours, and the THH doubles the half-life of DMT as shown by Dr. Mckenna, I can attest it does. You can keep re-does a 3rd time, as I did many hours later, and was still blown away. I have used it over 50 times in 2 years last year, and loved every minute of it.
I've taken oral Ayahuasca over 90 times in a decade, trust me when I say this is so much better, no nausea, no dizziness, as dmt is not travelling thru stomach.
More on sublingual Ayahuasca, my psychedelic of choice, I wrote this 2 years ago:

 mycotopia.net
mycotopia.net
1mg LSA + new aldehyde molecule discovery results in effects similar to 100ug LSD: https://www.shroomery.org/forums/showflat.php/Number/27850299/page/1
-------------------------------------------------------
Last and final updates 10.28.2022:
LSH + penniclavine combo psychedelic, natural LSD:
1) Zero nausea morning glory LSH + penniclavine combo tincture based on 2022 LSH study, natural LSD:

 mycotopia.net
mycotopia.net
or here: https://www.shroomery.org/forums/showflat.php/Number/27850299
Sublingual "under the tongue" Ayahuasca:
I use the sublingual HPBCD DMT weekly with zero nausea or dizziness used sublingually. Big difference compared to oral Ayahuasca which always make me feel nauseated, uncomfortable, dizzy like and with anxiety, with sublingual there is none of that. Take a look at L-dreamer's many experiences using the sublingual Ayahuasca several posts up. He rated it 10/10 just like me.
This sublingual Ayahuasca bi-weekly is so euphoric and very strong, zero nausea or dizziness, absolutely love her. All the visions, music enhancement, deep spiritual insights and teachings of oral Ayahuasca without the side effects: https://mycotopia.net/topic/111979-...to-3-times-better-than-dmt-salts-masks-taste/
or here: https://www.shroomery.org/forums/showflat.php/Number/27978052
------------------------------
I use the sublingual HPBCD DMT weekly with zero nausea or dizziness used sublingually. Big difference compared to oral Ayahuasca which always make me feel nauseated, uncomfortable, dizzy like and with anxiety, with sublingual there is none of that. Take a look at L-dreamer's many experiences using the sublingual Ayahuasca several posts up. He rated it 10/10 just like me.
 bluelight.org
bluelight.org
Update 7.24.2022, other topics you may be interested in:
1) How to make THH or tetrahydroharmine with pics, post #13:
https://mycotopia.net/topic/111610-hpbcd-dmt-sublingually-active-under-tongue/
THH can be made by converting harmaline from rue seeds in 1.5 hour using vinegar, zinc dust, magnetic stirrer and 10% janitorial ammonium hydroxide from the hardware store or on-line.
2) One shot HPBCD DMT Ayahuasca, masks taste and increases absorption many factors with pics, post #42:
https://mycotopia.net/topic/111610-hpbcd-dmt-sublingually-active-under-tongue/page-3
3) Journal: 50 Sublingual HPBCD DMT Ayahuasca journeys over a years time with pics, post #19:
https://mycotopia.net/topic/111790-...bcd-dmt-ayahuasca-journeys-over-a-years-time/
4) LSH extract tek: 500 Heavenly blue morning glory extract in 1oz everclear + 1 oz wine, imagine your best 2 hit LSD experience x 2
 bluelight.org
-------------------------------------------------------------------------------------------------------------------------------------------------------------
bluelight.org
-------------------------------------------------------------------------------------------------------------------------------------------------------------
-------------------------------------------------------------------------------------------------------------------------------------------------------------
4-24-2022
Thread summarized in 4 pages and continued here:
 bluelight.org
--------------------------------------------------------------------------------------------------------------------------------------------------------------
bluelight.org
--------------------------------------------------------------------------------------------------------------------------------------------------------------
--------------------------------------------------------------------------------------------------------------------------------------------------------------
--------------------------------------------------------------------------------------------------------------------------------------------------------------
2 minute formed HPBCD DMT liquid very bioavailable sublingually under tongue & outperforms DMT salts orally by many factors in personal trials, combo with tetrahydroharmine, Ayahuasca.
Here is the full journey report (150mg HPBCD DMT + 170mg harmine fb + 250mg THH or tetrahydroharmine freebase) all dissolved into 30ml (one-shot) hot water, added 150mg pure vitamin C powder so the freebase harmalas could dissolve, although only 100mg is needed:
Update 4pm 3/21/2022: I am writing this 1.5 hour later: took the 1-shot HPBCD DMT Ayahuasca (150mg HPBCD DMT, 250mg THH, 170mg harmine all dissolved into 30ml hot water) and it worked incredibly well! Phenomenal strength, for the 1st 10 minutes I held on tight as it felt like I was handling a high speed sports car, ego disintegrating & boundaries shattered.
For the first 10 minutes, open or closed eyes, didn't matter, saw same thing: colored neon geometrics on the surface of everything. Felt whole body high tryptamine frequency combined with the THH frequency vibration = amazing amplified whole body frequency, like being tuned into another spirit manifested alien world. It felt like a colored shining tractor beam was going to surround me and take me away, very 3-dimensional.
It was so powerful for 10 minutes, I had to close my eyes to find a happy place. Felt like the most powerful of my past Hawaiian psychotria experiences (40 grams leaf) with Caapi which I only ventured at this dose around 5 times (and all by accident, never meant to, as it is so strong) out of my 70 total Hawaiian psychotria journeys (most 30-35 grams), this was no different, exactly the same as the potent leaf, at the very high end for advanced Ayahuasca use.
Dennis Mckenna once said about one of his Ayahuasca journeys, that for the first 10 minutes it felt like he was riding an elevator to the top floor at high speed, this is how it felt for me, or like handling a high speed sports car.
...then it became at 10 minutes extremely enjoyable, infinite open eyed beauty, incredible closed eye morphing & dancing geometrics in wild neon purple, pink & green that lasted for another 35 minutes with closed eyes. With open eyes, saturated neon colors and neon colored rainbow reflections surrounded everything which glowed intensely with an inner divine light, music playing was just heavenly, incredible music enhancement, +5 Shulgin level experience, most powerful experience I've had in years....very strong for 1.5 hour, when it decreased in strength several notches.
I could not believe the open eyed beauty--phenomenal...this is my preferred method. WOW, unbelievable experience, high euphoria, deep headspace, profound spiritual insights, extremely visual, had the power of 40g Hawaiian psychotria to the highest degree. I experienced zero nausea, zero dizziness (so long as I keep my harmine dose below 200mg). Love, Love, Love.



-------------------------------------------------------------------------------------------------------------------------------
Part 0: 12 reasons pure THH or tetrahydroharmine rocks (this post #1 in middle)
Part 1: HPBCD complexed DMT experimental dosage, effects & duration, over 44 sublingual DMT experiences over a year's time (this post #1 at bottom with pics). Many times stronger than oral DMT. Updated 1-1-2022. (this post #1 near middle & bottom)
--> See the 5 hour brightly colored CEV trip report using 300mg oral THH once + 60mg sublingual HPBCD DMT + 35mg sublingual harmine fb x 2 re-doses every 1.5 hour at the very bottom of this post #1. HPBCD DMT kicks ass period. Most meaningful & intense CEV visionary experience of my life, and I've taken Ayahuasca x 70 times and high dose cactus tea over 200 times. Incredible music enhancement the whole time as well. <--
Part 2: L-dreamer's 2/4/2022 experience: "Sublingual DMT + oral THH - surprising and underrated" + Closing tips + Receptorome chart & explanation (post #5)
Part 3: 300mg Tetrahydroharmine (THH) teaching visions all by itself (post #17)
Part 4: Tetrahydroharmine receptorome similarities to mescaline; potentiates cactus & safety note [post #18]
Part 5: Chemist Patrick Arnold's HPBCD complexed prohormones for sublingual use (millions of dollars in sales) & bloodwork studies (post #15)
part 6: Dr. Narang: "with sublingual" or "under the tongue" better than buccal, gingival & palatal, absorption of drugs through the sublingual route is 3 to 10 times greater than oral route and is surpassed by hypodermic injection (post #20)
part 7: a little bit on my 70 Ayahuasca experiences, doses & visions (page 2, post #22)
part 8: New research: Morning glory contains 5 stimulating LSD-like drugs, soluble only in wine/alcohol, only sparingly soluble in water (page 2, post #40)
part 9: 20 minute visionary visit from a dead Aztec Shaman (page 3, post #42)
part 10: One way to make pure tetrahydroharmine (page 3, post #43)
part 11: From the archives of DMT world: How to easily extract 2.3g DMT from 170g bark using a 2 liter Erlenmeyer flask [page 3, post #48]
part 12: Out of print writings on the Divine Plant of the Incas, coca leaf visions...and writings on strong euphoria from coca leaf tea bags soaked in wine, forming orally active cocaethylene in the liver, discovered in 1994. Explains the popularity of Vin Mariani (coca leaf soaked wine) with both popes, Thomas Edison and countless celebrities. (page 3, post #49)
part 13: THH + mushrooms report from JKW (page 3, post #50)
part 14: Tetrahydroharmine + 1-acetaldehyde LSD (similar to ALD-52) combo, like high dose mescaline (page 3, post #56)
part 15: Multiple encounters with death and depression & 80mg DMT complexed to 560mg HPBCD oral Ayahuasca report
[page 3, post #58]
part 16: Late March 2022 experience: Oral Ayahuasca: 250mg oral THH fb + 160mg oral harmine fb + 140mg HPBCD DMT, THH and harmine in gelatin capsules, 140mg HPBCD DMT dissolves from off spoon instantly into 1 shot glass of 125 degree F water, transparent color. Many times more potent than freebase or DMT salts used orally. HPBCD not only helps to mask the taste of the nasty freebase DMT as it goes down but improves absorption into intestinal tissues many factors over. (page 4, post 80)
---------------------------------------------------------------------------------------------------------------------------------------------------
10.18.2021 update, (see page 3, post #56 for details)
Tetrahydroharmine + 1-acetaldehyde LSD (similar to ALD-52) combo, like high dose mescaline:
In closing, I'm going to post what I believe to be a revolutionary psychedelic combination, and it's dirt cheap compared to the rare and very expensive cactus...but it's just as long-lasting, profound, highly euphoric, visual, neon-colorful, music-enhancing & super deep head space, with zero-anxiety as two feet of fat bridgesii.
300mg THH + 250ug 1-acetaldehyde LSD report (2oz fresh cold sherry wine morning glory extract can substitute as well)
1) The combo of 300mg THH + 1-acetaldehyde LSD makes the beauty & aesthetic enhancement way stronger than LSD alone. Same "over the top" beauty enhancement as high dose cactus tea.
2) The music sounds much better than LSD alone, it feels very much like when you combine mescaline with LSD, as THH is like the beta-carboline version of mescaline.
3) The combo is highly visual & neon-colorful with open eyes, with each of the 12 trips spaced two weeks apart experienced so far have seen neon-red-greens, neon-orange-blues, and even neon-purple-yellows, supercolorful just like high-dose cactus tea.
3) Very beautiful combination.
4) This 300mg THH + 250ug 1-acetaldehyde LSD combo is one of my absolute favorites, have since used it every 2 weeks x 12 times now. No re-doses necessary as the THH has a 10.5 hour half life with peak at 5.25 hours. Very powerful: Lasts all evening, infinitely beautiful. I've consistently reached +5 Shulgin level strength every time, very life changing experience every time. Super deep head space, Divine to the extreme, heavenly mescaline-like spiritual euphoria for hours on end, no words to describe.
Note: THH is NOT an MAOI, she (feminine spirit) is a psychedelic SRI or serotonin reuptake inhibitor just like the following psychedelic serotonin reuptake inhibitors: mescaline, LSD, shrooms, ibogaine.
Make sure your THH is pure and not contaminated with unconverted harmaline (which is a RIMA/maoi). Dab some THH on a wet cue tip, rub on paper plate, hold under blacklight, if it glows blue you have THH, if any green glow, you have harmaline in it, keep in mind harmine also glows blue too though.
-------------------------------------------------------------------------------------------------------------------------------------------------
(0) Part 0: 12 reasons pure THH or tetrahydroharmine rocks
-------------------------------------------------------------------------------------------------------------------------------------------------
1. Part 10 of this paper: shows how to convert harmaline to pure THH in 1.5 hour for the first time (very fast) with 75% yield. TIHKAL THH entry also achieved 75% yield. Post also shows how to check the blue glow under blacklight to make sure it is pure. Any green in the glow means you still have un-converted harmaline, but follow instructions and you won't have any unconverted.
1. Dennis Mckenna Ph.D: page 115:
In my experience, THH doubles the half-life of DMT, so when used sublingually or orally, you get a full strong 90 minutes out of it with long afterglow.
2. She is in the same beta-carboline family as ibogaine. She is the 2nd highest alkaloid in Caapi. She has a 10.5 hour half-life with peak at 5.25 hours.
3. DMT only colors are subdued and dark, but THH brightens the DMT visuals: out of this world impossible bright neon colors are a trait of high dose oral tetrahydroharmine + moderate dose 60 to 70mg+ sublingual or oral HPBCD DMT: neon red-greens, neon orange-blues, neon purple-yellows.
4. DMT does not block serotonin on it's own, but THH does...this results in not only stimulation but euphoria in combo with the DMT: and real Ayahuasca visions become apparent...important teamwork. Ibogaine, LSD, mescaline, shrooms, 5-meo-dmt, bufotenin in Amazonian snuffs, all block serotonin, THH blocks serotonin.
5. THH has numerous similarities to mescaline, she is like the beta-carboline version of mescaline, few people have used her over 100mg. I have seen the receptorome chart for THH vs. mescaline. She not only blocks serotonin like mescaline, but agonizes all 3 adrenal receptors A1-A3 associated with beauty and aesthetic enhancement, just like mescaline. Beauty enhancement is "over the top" when THH is included, she is diamondlike shimmering in her beauty.
Actresses on TV will look like dazzling glowing super-colorful cartoon versions of themselves (just like with high dose cactus tea) only if you include the THH. Researchers have called THH the "tryptamine of the beta-carboline world" and rightly so.
6. THH is found in average 150mg in a cup of Caapi based Ayahuasca tea, when 2 cups are drank by some of the more advanced members for evening at the vegetals (UDV, Santo Daime, Shuar Indian) people are consuming around 300mg of THH.
7. Music will only sound bad-ass incredible if you include from 150mg to 300mg oral THH with your sublingual or oral DMT.
8. This pure THH at 300mg all by herself is extremely visual, she's an isomer of a hormone like substance made in the brain naturally.
9. The entry in TIHKAL for 300mg THH is completely wrong, where the unexperienced person compares it to the effects of 100mg harmaline. She is nothing at all like harmaline, and like 69ron once said about the person's comment in TIHKAL, he or she would not be able to tell their ass from their elbow. I agree, what complete nonsense. Dr. Shulgin wrote that he never got the chance to try THH, but wrote that more studies on it are "badly needed."
10. professor8 (found here from 11/1/2010 he writes like a poet w/special powers of imagination & expression):
11. Trips (from here on 12/2/2011):
Espiridion:
The world is moving in the direction of the Left Brain: technology and science. What the world needs is to move in the direction of Right Brain development: empathy, spirituality, connectedness. Compounds like tetrahydroharmine in Caapi could be said to improve emotional intelligence. Is this component of caapi a smart-nutrient for the right side of the brain? you be the judge.
At 300mg of THH all by itself, there are heavy open-eyed tracers like lightening flashes, and hours of teaching closed eye visions that start with colored sparkles and fireworks (red, green, yellow, blue) that dart around and progress into full-fledged way-beyond 4k visions with eyes closed that are not only static but often animated like slow and high speed movies, but all one monochrome color like green or blue for me, when you add DMT, the visions then become colored and patterning on animals for example will display their associated colors, DMT also adds on to or builds on top the THH visions, expanding them, but the teachings and insights & visions are credited to the Vine, just as Gayle Highpine writes in linked paper:
12. Gayle Highpine (Ayahuasca researcher):
Tetrahydroharmine or THH ranks very high on the "periodic psychedelic table" among all the known entheogens for inducing realistic way beyond 4k monochrome teaching visions for hours...adding even small amounts of DMT brightens and colorizes the visions, example: reptiles, birds & animals such as serpents/snakes/toucans/parrots/jaguars with patterning show their respective associated colors. Many times I have viewed multi-colored serpents, birds & jaguars several times over hour long CEV periods, serpents are the manifest spirit of Ayahuasca.
Daniel Pinchbeck "Breaking Open the Head" (Daniel also states in his book, that Ayahuasca is his favorite entheogen):
Graham Hancock, "Supernatural", pg 428:
Gayle Highpine (Ayahuasca researcher):
Psychedelia, page 61:
Part 1: HPBCD complexed DMT experimental dosage, effects & duration
---------------------------------------------------------------------------------------------------------------------------------------------------
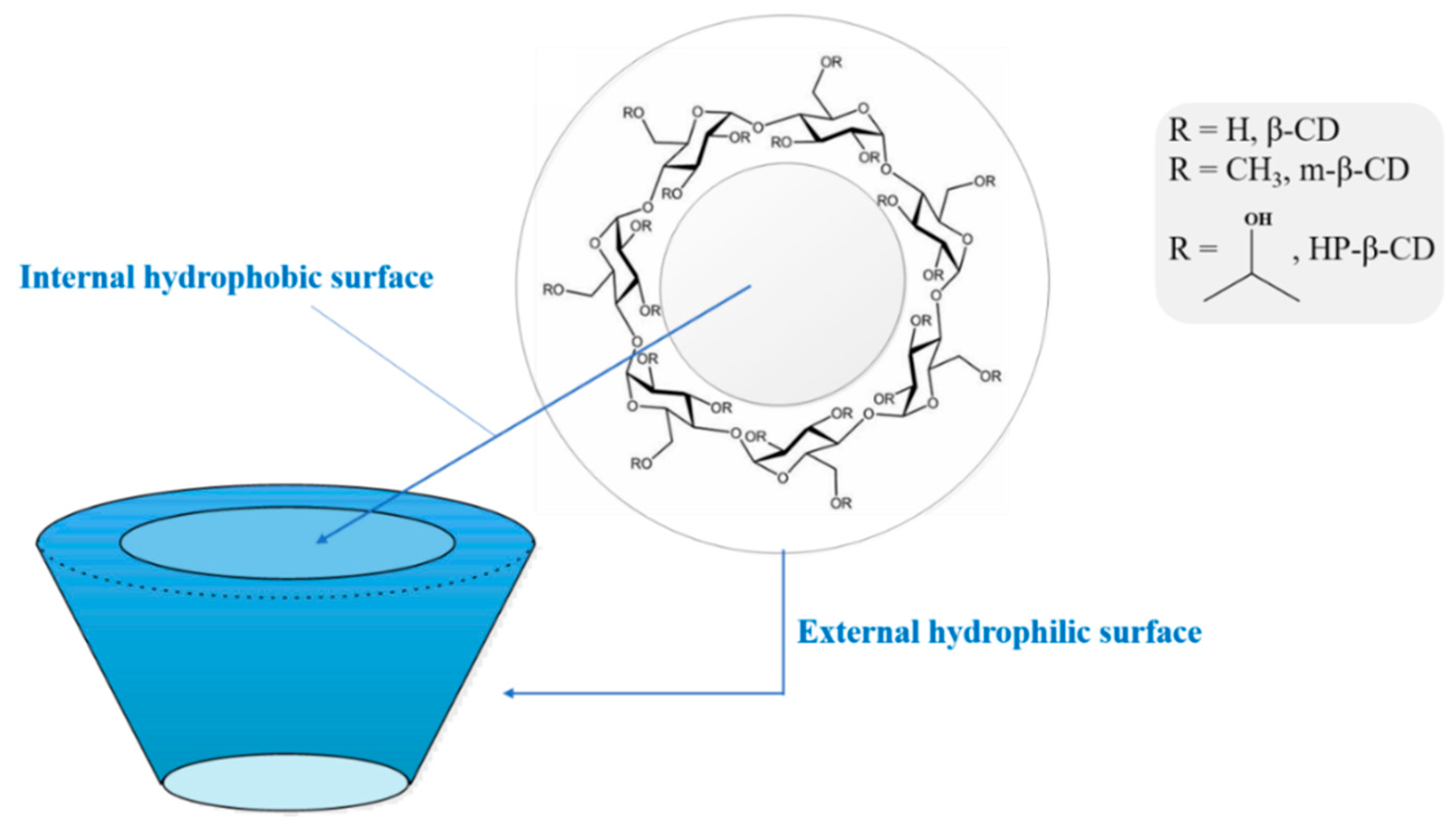
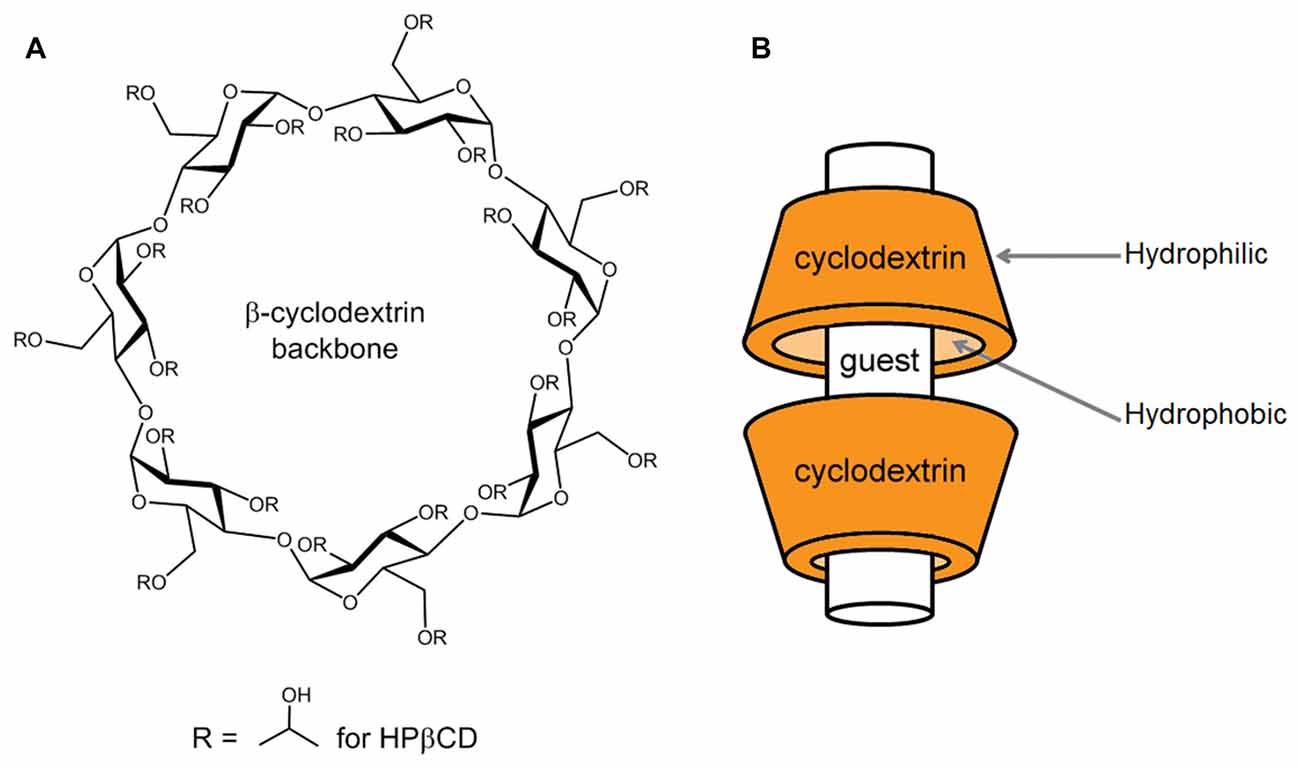
How this works: the Hydroxy propyl beta cyclodextrin molecule (HPBCD) has an inner hydrophobic cavity (repels water) which attracts & traps freebase molecules like DMT freebase inside it's tornado shaped cone. The outer cavity is hydrophilic (likes water) and thus makes the DMT molecule water-soluble. HPBCD, being a polysaccharide derived from the enzymatic degradation of starch, improves the penetration of the DMT freebase many factors over (studies show x 4 factors or 400 percent into the sublingual mucosa under the tongue.
HPBCD, being a potent polysaccharide derived from the enzymatic degradation of starch, improves the penetration of the DMT freebase many factors over (studies show x 4 factors or 400%) into the sublingual mucosa under the tongue. HPBCD then releases the DMT as the potent freebase into the bloodstream once it crosses the sublingual mucosa, these reasons explain it's potency.
Keep in mind using DMT salts sublingually does not work, and there is no penetration enhancement unless HPBCD is used to complex DMT in the freebase form only. The cyclodextrins have toroidal shapes, with the larger and the smaller openings of the toroid exposing to the solvent secondary and primary hydroxyl groups respectively.
You can order HPBCD from China no matter where you live as it is legal, and if you google "Europe + HPBCD" there are a couple places that sell it as well. It is very common in the USA from *mazon or auction sites.
In closing, complete instructions and pictures all updated for 1-1-2022 on sublingual HPBCD DMT & summary of over 44 sublingual experiences in a year's time (bottom of post #1), these experiences all must stronger than oral DMT by many factors. Zero nausea, zero dizziness, zero anxiety or un-easy feelings, deep head space, highly visual, strong open eyed and CEV's of spinning & dancing geometrics & real Ayahuasca visions, incredible music enhancement, way over the top open eyed beauty, neon colorful & highly euphoric, see trip reports bottom of post #1...will continue to use for the rest of my life, highly recommend: https://www.dmt-nexus.me/forum/default.aspx?g=posts&t=96861
---------------------------------------------------------------------------------------------
Complete instructions and pictures all updated for 1-1-2022 on sublingual HPBCD DMT & summary of over 44 sublingual experiences in a year's time, these experiences all much stronger than oral DMT by many factors. Zero nausea, zero dizziness, zero anxiety or un-easy feelings, deep head space, highly visual, strong open eyed and CEV's of spinning & dancing geometrics & real Ayahuasca visions, incredible music enhancement, way over the top open eyed beauty, neon colorful & highly euphoric, see trip reports bottom of post #1...will continue to use for the rest of my life, highly recommend: https://www.dmt-nexus.me...aspx?g=posts&t=96861
My procedure:
1) place 60mg of DMT onto a spoon
2) add 1:1 molar ratio of host drug to HPBCD powder, this means 1:7 mg ratio DMT to HPBCD, use a 1:8 mg ratio DMT to HPBCD if you are using the 2-Hydroxypropyl-β-cyclodextrin.
3) this means 60mg dmt placed on spoon, then add 420mg of HPBCD on top DMT, use 480mg HPBCD if you are using the 2-Hydroxypropyl-β-cyclodextrin.
4) add 10 drops of very hot near boiling water to the mix from a nearby microwaved coffee mug for DMT doses of 90mg or below, use 12 drops of boiling hot water to mix DMT doses over 90mg (such as 100 to 120mg). 60mg DMT = +3 Shulgin level strength, 90 to 120mg = +5 Shulgin level life changing strength.
5) Knead or crush the HPBCD powder into the dmt using the end of a spoon for 2 minutes, scrape & mix everything back and forth hard using your muscles. This is how scientist pre-pare these complexes by kneading.
6) Optional (you do not have to do this): Hold a lighter far away from under the spoon to heat up spoon for around 20 seconds or so, then pull flame away, this seems to aid dissolution or dissolving after heating up a slight bit, mix the contents a little bit more before using.
7) Take 150 to 300mg tetrahydroharmine orally around 45 minutes before...and take 200mg harmine orally around 45 minutes to 1 hour before...if you don't take harmine orally, then place 25 to 35mg of freebase harmine under your tongue. Then place bottom of your tongue onto HPBCD complexed DMT spoon, the HPBCD DMT glob will all adhere as HPBCD powder forms sticky complexes. Be sure to take the 35mg harmine and HPBCD DMT under tongue all at the exact same time in order to activate the DMT strongly.
Hold under tongue for 10 to 12 or 15 minutes depending on dosage, hold under tongue the whole time to trap sticky liquid complex in the sublingual mucosa. Be sure to use bottom end of tongue to lick any off spoon that is left behind, you want to get it all.
At end of 15 minutes, spit out any saliva into a cup instead of swallowing. Gently relieve any saliva during the 15 minute period as comfort dictates by leaning your head forward and spitting into a cup, but keep your tongue pressed down any time you relieve saliva. There is a mild to moderate sting felt but it's so worth enduring for the effects which begin in 22 minutes or 1/2 the time of an oral dose (44 minutes). The sting does not bother me at all. The tongue is completely fine afterwards (no burn), and it's as if nothing happened the next day.
So long as the HPBCD DMT is used sublingually, there is zero nausea, zero dizziness, no delirium, crystal clear clarity like mescaline...there is something about DMT going thru the stomach that tends to induce nausea and weird uncomfortable strange feelings, just as L-dreamer mentions in post #2 of this thread at the very beginning. Same thing with me. It's also no where near as potent as sublingually used HPBCD DMT. Sublingual HPBCD DMT is at least x 5 times stronger than oral DMT.
---------------------------------------------------------------------------------------------
The combination of THH + DMT simulates true Ayahuasca, but there is zero nausea, zero dizziness, zero queasiness since there is no harmine or DMT going thru stomach and intestines. 22 minutes in there are heavy CEV's of spinning colored geometrics, visions of ancient architecture, animals, aliens, you name it, She seems to tap into the Akashic record of the ether in the Universe, where all past, present, and future is known. Open eyed profound beauty, music sounds incredible.
The sublingual application is several factors stronger than oral DMT, and I've used oral DMT dozens of times. I tolerate this very well compared to oral DMT, extremely pleasurable experience, high spiritual euphoria.
I don't like oral DMT or oral harmine, both make me dizzy, give me anxiety and un-easy feelings, and just keep saying "I want this to end". But the sublingual method is a complete 180 degree, feels great like mescaline all evening long.
Oral 300mg THH taken 45 minutes earlier + sublingual 60 to 120mg HPBCD DMT combined with sublingual 35mg harmine fb journeys (over 44 of them over a year's time). This counts the two subsequent re-doses every 1.5 hour for the evening, for a 4.5 hour total strong journey with super-long afterglow.
First of all there was a deep head space, profound spiritual insights and revelations. I was seeing curtains of visuals in the doorway, closed eye neon spinning & dancing geometrics, music enhancement incredible, out of this world impossible neon colors (like neon yellow-purple) flashing on the walls, some times these colors on the walls would break up into fine lines like lazer beams, and paste themselves like hundreds of beams broadcast on the walls, concentric circle rings in the air, powerful lightening like tracers.
Open eyed beauty so over the top & infinite, actresses in movies looked like glowing, dazzling, super-colorful, cartoon versions of themselves, just like with high dose cactus tea, also zero anxiety just like mescaline. Euphoria was powerful, just like with high dose cactus tea.
Again with the sublingual: pupil dilation maxed out, strong tryptamine body buzz high frequency, heavy CEV imagery, open eyed beauty profound, music sounds incredible.
On my very first 300mg oral THH + sublingual 60mg HPBCD DMT combined with 35mg sublingual harmine fb trip with 2 sublingual re-doses at each 1.5 hour mark (had not used DMT in several months)....all the way till 5am in the morning I was seeing closed eye visions of slow and high speed movies...I saw brightly colored serpents, dungeons I traveled thru, many Mesoamerican pyramids, women of incredible beauty, Japanese landscapes, dancing geometrics, many different animals on a rotating globe, walking on the planet-like globe as it spun, hundreds of visions like slow and high-speed movies over the course of many hours.
I wore headphones and listened to music the whole time, as the music sounded just like if I had taken a very strong cactus tea.
I saw the interiors of many magnificent homes, exposed like a camera flash went off, then off to the next home interior, bizarre alien looking creatures, I saw ancient ruins but they were seen as they were before they fell apart. All sorts of architectural wonders appeared that I could not make out exactly what time period they were from.
All the visions were enchanting & manifested incredible beauty. The multi-colored beautiful serpents kept appearing several times in different forms, as if they have some prominence to do with it all, two of them had shining skin covered in gold scales and intertwined like DNA, reminds me of the Aztec quetzalcoatl myth, the "serpent of precious feathers."
...all of these visions were brightly colored due to the sublingual DMT/harmine and oral THH combo all night long..it was one of the most powerful psychedelic experiences of my life...and I've taken Ayahuasca x 70 times, cactus 200 times, etc...I have never had over 5 hours of non-stop CEV visions anything close to what I saw that first night.
The visions inspired me to buy a book on the Aztec myth of "Quetzalcoatl, the serpent of precious feathers", as I feel somehow this entity is a "teacher to mankind". I saw the brightly colored serpents many times in the 5 hours of visions, and now I understand why they are so commonly reported in Ayahuasca journeys.
They seem to possess divine knowledge that humans were not supposed to have been privileged to, but the serpents gifted this knowledge to humankind.
Recently found a 1.5 hour video on Amazon prime entitled "Ancient Alien Origins" which is all about this ancient alien flying serpent or dragon entity which is found in all religions of the world & "BAM, Builders of the Ancient Mysteries".
Return of the Serpent & of Eden:

 earthmedicine2015.wordpress.com
hxxps://earthmedicine2015.wordpress.com/2016/01/29/return-of-the-serpent-of-eden/
earthmedicine2015.wordpress.com
hxxps://earthmedicine2015.wordpress.com/2016/01/29/return-of-the-serpent-of-eden/
https://en.wikipedia.org/wiki/Mind_at_Large
300mg of tetrahydroharmine (THH) is equated to the (CEV) power of 100mg harmaline, but without all the nausea and dizziness. It glows blue under blacklight, like LSD or psilocin & has a metallic-like lingering taste with a 10.5 hour half-life.
"Cyclodextrins used as excipients" pdf, European Medicines Agency, Oct 9, 2017: page 5 of 16 under "Oral products, Kinetics":
This pharmacology has been used to potentiate these freebase drugs ORALLY as well -- in my experience, this results in an Ayahuasca experience that is strong in potency. Example: HPBCD improves oral absorption profile for Ofloxacin, a second generation fluroquinolones by 54 to 89 percent.
I've used HPBCD complexed DMT orally three times stirred into a 1oz hot water tea (it dissolves instantly) with 150 to 300mg THH + 160 to 200mg harmine with a bit of citric acid to help the harmalas dissolve, and it's also way more potent than normal DMT freebase or salts used orally, very reminiscent of my over 70 Hawaiian psychotria brewed leaf teas, very strong & all encompassing. Trip report on page 2, post #25.
But I vastly prefer the oral 150 to 300mg THH + sublingual Ayahuasca (35mg sublingual harmine fb + 60 to 100mg sublingual HPBCD DMT all taken at exact same time under tongue), many times stronger than oral DMT, and zero dizziness or un-easy feelings for me due to only 35mg harmine being used. Highly recommend for sensitive individuals like myself. Very strong & feels great all evening like mescaline, heavenly.
"Sublingual mucosa as a route for systemic drug delivery" by Narang & Sharma 2010:
https://innovareacad...Suppl2/1092.pdf
As you can see from this sublingual viagra study, even 50 to 100mg doses can be administered under the tongue, the authors noting that less of the drug was required, and that it began working in only one half the normal time of an oral dose:
"The start of pharmacological activity after sublingual administration of sildenafil citrate in 30 patients affected by erectile dysfunction." by Siati & Franzolin 2003:
https://pubmed.ncbi....h.gov/12741340/
Tetrahydroharmine on it's own will also yield the same type visions as harmaline, it just takes more of it. For example, around 300mg of THH will yield the same visions as about 100mg harmaline...even if the THH dose is split in two over several hours, the visions will still be apparent some time after the 2nd dose takes effect, the doses are additive.
THH in the caapi also seems to strongly activate the right hand hemisphere of the brain-- the side that performs tasks that have do with creativity and the arts, feelings, visualizations, imagination, holistic thinking & intuition, empathy, spirituality & connectedness. Researchers found that the right side of the brain lit up in brain scans of people who took LSD, mescaline, or mushrooms. This includes tetrahydroharmine. The world is largely moving in the direction of the Left Brain: technology and science. What the world needs is to move in the direction of Right Brain development.
Quote from TIHKAL by Dr. Shulgin "More studies on tetrahydroharmine are absolutely imperative."
References:
Dennis Mckenna Ph.D:
Jamie, posted : 11/23/2012 8:29:28 PM:
Dr. Narang:
"Cyclodextrins used as excipients" pdf, European Medicines Agency, Oct 9, 2017:, under "Kinetics":
Pic 1: How to mix the DMT, HPBCD, and drops of very hot water on a spoon.
Pic 2: 1kg container of HPBCD from China for cheap. 2-hydroxy-propyl-beta-cyclodextrin is the more common form available, works the exact same.
Pic 3: Sublingual mucosa under tongue only 100 to 200 microns thick.
Pic 4: 300mg pure THH for oral use in weigher, 35mg harmine fb for sublingual use next to it, 60mg dmt fb in spoon, 420mg HPBCD white powder above it, coffee mug of near boiling hot water, medicine dropper pipette, Bic lighter.
Pic 5: 60mg DMT was covered with x 7 or 420mg HPBCD powder and 10 drops of near boiling hot water from a nearby coffee mug was added, and all mashed and stirred well for 2 minutes, then Bic lighter held under spoon until the solution just started to boil (20 seconds or so), then pull flame away, notice how well the DMT complexed inside the HPBCD.
Take 150 to 300mg tetrahydroharmine 45 minutes earlier orally, place 35mg harmine under tongue, then place tongue on to HPBCD complexed DMT spoon, it will all adhere for sublingual use. Journey starts in 22 minutes after application and lasts x 90 minutes, at which time you can re-dose x 2 more times, each dose just as strong as 1st dose for a 4.5 hour long strong journey with super-long afterglow. Instructions, 5 hour CEV trip report and 9 pics here on post #1.
--------------------------------------------------------------------
Stay true to yourself, Love, Peace and Music
http://www.friskyradio.com




 Sublingual mucosa as a route for systemic drug delivery (2).pdf 240.7KB 3 downloads
Sublingual mucosa as a route for systemic drug delivery (2).pdf 240.7KB 3 downloads
 Thermodynamic properties of hydroxypropyl Beta cyclodextrin and guest interaction, a survey of recent studies, 2021.pdf 854.1KB 6 downloads
Thermodynamic properties of hydroxypropyl Beta cyclodextrin and guest interaction, a survey of recent studies, 2021.pdf 854.1KB 6 downloads
questions-answers-cyclodextrins-used-excipients-medicinal-products-human-use_en.pdf 273.35KB 5 downloads
 HPBCD solubility enhancement table for 68 drugs.pdf 61.76KB 5 downloads
HPBCD solubility enhancement table for 68 drugs.pdf 61.76KB 5 downloads
Sublingual Ayahuasca, my psychedelic of choice: https://www.shroomery.org/forums/showflat.php/Number/28189371
hxxps://www.shroomery.org/forums/showflat.php/Number/28189371 (change hxxp to http to link)
I've actually decided to save my bridgesii only for when I go outdoors to the waterpark with my family or in nature at the river. I always get a mild hangover from cactus that makes me extra tired, and I end up sleeping too much of the next day away, and my beautiful, funny and smart wife gets aggravated with me
I actually went back to taking 200mg tetrahydroharmine + 200mg harmine orally at one time in a capsule, then 1 hour later when I feel it working, I place the HPBCD DMT under my tongue and wait for it dissolve which happens 100% in 15 minutes. It is no different from high dose cactus in effects for 1.5 hour with an incredible afterglow.
Sublingual Ayahuasca directions:
1) Take 200mg harmine freebase and 200mg pure THH freebase orally in a capsule
How to make pure THH or tetrahydroharmine here, post #13: https://mycotopia.net/topic/111610-hpbcd-dmt-sublingually-active-under-tongue/
2) 1 hour later: Place 120mg DMT on a spoon
cover with x 7 or 840mg Hydroxy propyl beta cyclodextrin or HPBCD also use the 2-Hpbcd, same in effect. This keeps it at a 1:1 molar ratio, as HPBCD (1300 g/mol, is x7 bigger in mol/g weight by dmt (188 g/mol).
3) add 10 drops of hot water from a microwaved coffee mug
4) mix and scrape it all back and forth for 1 to 2 minutes on the spoon using the end of another spoon, the complex is formed with absorption into the sublingual mucosa x 4 normal or 400% increase studies show.
5) Place bottom side of tongue onto spoon, the HPBCD DMT complex will all adhere, as HPBCD forms sticky complexes like sugar. Now hold under your tongue for 15 minutes, if at any time you feel too much saliva form, simply tilt head forward and spit out the extra saliva into a cup, but always keep tongue pressed down.
How this works: the oral harmine you took works in the brain to prevent the "zapping of dmt" by mitochondria located within the cells of the brain according to Dr. Mckenna, mao is reduced in the brain for several hours as harmine has a half life of around 1 to 3 hours. So once the dmt is absorbed sublingually, the harmine working in the brain prevents it from breaking down, and the THH doubles it's half life. The THH is critical as it is a major alkaloid of Caapi, a feminine teaching spirit who causes visions all on her own, she is the guide and teacher for the experience. She also blocks serotonin which causes mild stimulation, dmt does not block serotonin.
There is a mild sting but it is so worth the wait. The HPBCD being such a large molecular weight is not absorbed by the mucosa but does facilitate the transfer of the DMT into the bloodstream as the potent freebase by opening up of "tight junctions" as research into HPBCD shows. The HPBCD ends up in the saliva you spit out after 15 minutes. The mucosa under tongue is also only 100 to 200 micrograms thick, it is rich for this activity and many times more potent than normal oral absorption according to Dr. Narang.
When you mix it on the spoon, use your muscles to scrape, mix and knead it all together. This is how scientist prepare these complexes, by kneading.
1.5 hour incredible experience +5 shulgin in intensity and euphoria and beauty....beautiful geometrics overlaid all surfaces, music beyond heavenly, just incredible music enhancement...actresses on tv look like goddesses.
The colors omg are just neon like and out of this world, so beautiful, the shimmering of everything is beyond belief. The beauty with open eyes is divine and infinite. The interconnection of all things is seen, and very deep spiritual insights and healing. I walked out in nature and lost myself in the beauty.
None of the negatives of oral Ayahuasca, as oral DMT makes my very nauseated and uncomfortable, this is all positives...very euphoric and straight to bloodstream as the potent freebase.
Way better than LSD.....and just as good as high dose cactus tea. I had an experience tonight that blew my mind, this is my method to use every weekend for the rest of my life, Sublingual Ayahuasca is a great way to stay psychedelic for the rest of your life. I re-dosed more HPBCD DMT at the 1.5 hour point, and the fun continued for another 1.5 hour, as the oral harmine has a half life of from 1 to 3 hours, and the THH doubles the half-life of DMT as shown by Dr. Mckenna, I can attest it does. You can keep re-does a 3rd time, as I did many hours later, and was still blown away. I have used it over 50 times in 2 years last year, and loved every minute of it.
I've taken oral Ayahuasca over 90 times in a decade, trust me when I say this is so much better, no nausea, no dizziness, as dmt is not travelling thru stomach.
More on sublingual Ayahuasca, my psychedelic of choice, I wrote this 2 years ago:

One-shot hpbcd dmt ayahuasca and sublingual hpbcd dmt ayahuasca, absorbs 2 to 3 times better than DMT salts, masks taste - Botanicals
Growing Magic Mushrooms, Mushroom spores, Ayahuasca, Magic Mushroom, Cultivation, Magic Mushroom Cultivation, Psilocybe Mushrooms, Cactis and Cannabis, including research, legislation, media coverage, bibliography and lots of links
1mg LSA + new aldehyde molecule discovery results in effects similar to 100ug LSD: https://www.shroomery.org/forums/showflat.php/Number/27850299/page/1
-------------------------------------------------------
Last and final updates 10.28.2022:
LSH + penniclavine combo psychedelic, natural LSD:
1) Zero nausea morning glory LSH + penniclavine combo tincture based on 2022 LSH study, natural LSD:

New research: Morning glory contains 5 stimulating LSD-like drugs, soluble only in wine/alcohol, only sparingly soluble in water. - Page 8 - Botanicals
Growing Magic Mushrooms, Mushroom spores, Ayahuasca, Magic Mushroom, Cultivation, Magic Mushroom Cultivation, Psilocybe Mushrooms, Cactis and Cannabis, including research, legislation, media coverage, bibliography and lots of links
or here: https://www.shroomery.org/forums/showflat.php/Number/27850299
Sublingual "under the tongue" Ayahuasca:
I use the sublingual HPBCD DMT weekly with zero nausea or dizziness used sublingually. Big difference compared to oral Ayahuasca which always make me feel nauseated, uncomfortable, dizzy like and with anxiety, with sublingual there is none of that. Take a look at L-dreamer's many experiences using the sublingual Ayahuasca several posts up. He rated it 10/10 just like me.
This sublingual Ayahuasca bi-weekly is so euphoric and very strong, zero nausea or dizziness, absolutely love her. All the visions, music enhancement, deep spiritual insights and teachings of oral Ayahuasca without the side effects: https://mycotopia.net/topic/111979-...to-3-times-better-than-dmt-salts-masks-taste/
or here: https://www.shroomery.org/forums/showflat.php/Number/27978052
------------------------------
I use the sublingual HPBCD DMT weekly with zero nausea or dizziness used sublingually. Big difference compared to oral Ayahuasca which always make me feel nauseated, uncomfortable, dizzy like and with anxiety, with sublingual there is none of that. Take a look at L-dreamer's many experiences using the sublingual Ayahuasca several posts up. He rated it 10/10 just like me.
Tryptamines - One-shot hpbcd dmt ayahuasca and sublingual hpbcd dmt ayahuasca, absorbs 2 to 3 times better than DMT salts, masks taste
Sublingual Ayahuasca, my psychedelic of choice: https://www.shroomery.org/forums/showflat.php/Number/28189371 Compilation of panaeolus cyanescens or pan cyan experiences & combo with THH or tetrahydroharmine, like organic psilocin LSD, extremely visual, music enhancing & super euphoric...
Update 7.24.2022, other topics you may be interested in:
1) How to make THH or tetrahydroharmine with pics, post #13:
https://mycotopia.net/topic/111610-hpbcd-dmt-sublingually-active-under-tongue/
THH can be made by converting harmaline from rue seeds in 1.5 hour using vinegar, zinc dust, magnetic stirrer and 10% janitorial ammonium hydroxide from the hardware store or on-line.
2) One shot HPBCD DMT Ayahuasca, masks taste and increases absorption many factors with pics, post #42:
https://mycotopia.net/topic/111610-hpbcd-dmt-sublingually-active-under-tongue/page-3
3) Journal: 50 Sublingual HPBCD DMT Ayahuasca journeys over a years time with pics, post #19:
https://mycotopia.net/topic/111790-...bcd-dmt-ayahuasca-journeys-over-a-years-time/
4) LSH extract tek: 500 Heavenly blue morning glory extract in 1oz everclear + 1 oz wine, imagine your best 2 hit LSD experience x 2
Lysergamides - Zero nausea morning glory LSH tincture based on 2022 LSH study, natural LSD
Compilation of panaeolus cyanescens experiences & combo with THH or tetrahydroharmine, like organic psilocin LSD: https://bluelight.org/xf/threads/compilation-of-panaeolus-cyanescens-experiences-combo-with-thh-or-tetrahydroharmine-like-organic-psilocin-lsd.927995/...
 bluelight.org
bluelight.org
-------------------------------------------------------------------------------------------------------------------------------------------------------------
4-24-2022
Thread summarized in 4 pages and continued here:
Tryptamines - Journal: 50 Sublingual HPBCD DMT Ayahuasca journeys over a years time
Compilation of panaeolus cyanescens or pan cyan experiences & combo with THH or tetrahydroharmine, like organic psilocin LSD, extremely visual, music enhancing & super euphoric...
--------------------------------------------------------------------------------------------------------------------------------------------------------------
--------------------------------------------------------------------------------------------------------------------------------------------------------------
2 minute formed HPBCD DMT liquid very bioavailable sublingually under tongue & outperforms DMT salts orally by many factors in personal trials, combo with tetrahydroharmine, Ayahuasca.
Here is the full journey report (150mg HPBCD DMT + 170mg harmine fb + 250mg THH or tetrahydroharmine freebase) all dissolved into 30ml (one-shot) hot water, added 150mg pure vitamin C powder so the freebase harmalas could dissolve, although only 100mg is needed:
Update 4pm 3/21/2022: I am writing this 1.5 hour later: took the 1-shot HPBCD DMT Ayahuasca (150mg HPBCD DMT, 250mg THH, 170mg harmine all dissolved into 30ml hot water) and it worked incredibly well! Phenomenal strength, for the 1st 10 minutes I held on tight as it felt like I was handling a high speed sports car, ego disintegrating & boundaries shattered.
For the first 10 minutes, open or closed eyes, didn't matter, saw same thing: colored neon geometrics on the surface of everything. Felt whole body high tryptamine frequency combined with the THH frequency vibration = amazing amplified whole body frequency, like being tuned into another spirit manifested alien world. It felt like a colored shining tractor beam was going to surround me and take me away, very 3-dimensional.
It was so powerful for 10 minutes, I had to close my eyes to find a happy place. Felt like the most powerful of my past Hawaiian psychotria experiences (40 grams leaf) with Caapi which I only ventured at this dose around 5 times (and all by accident, never meant to, as it is so strong) out of my 70 total Hawaiian psychotria journeys (most 30-35 grams), this was no different, exactly the same as the potent leaf, at the very high end for advanced Ayahuasca use.
Dennis Mckenna once said about one of his Ayahuasca journeys, that for the first 10 minutes it felt like he was riding an elevator to the top floor at high speed, this is how it felt for me, or like handling a high speed sports car.
...then it became at 10 minutes extremely enjoyable, infinite open eyed beauty, incredible closed eye morphing & dancing geometrics in wild neon purple, pink & green that lasted for another 35 minutes with closed eyes. With open eyes, saturated neon colors and neon colored rainbow reflections surrounded everything which glowed intensely with an inner divine light, music playing was just heavenly, incredible music enhancement, +5 Shulgin level experience, most powerful experience I've had in years....very strong for 1.5 hour, when it decreased in strength several notches.
I could not believe the open eyed beauty--phenomenal...this is my preferred method. WOW, unbelievable experience, high euphoria, deep headspace, profound spiritual insights, extremely visual, had the power of 40g Hawaiian psychotria to the highest degree. I experienced zero nausea, zero dizziness (so long as I keep my harmine dose below 200mg). Love, Love, Love.
-------------------------------------------------------------------------------------------------------------------------------
Part 0: 12 reasons pure THH or tetrahydroharmine rocks (this post #1 in middle)
Part 1: HPBCD complexed DMT experimental dosage, effects & duration, over 44 sublingual DMT experiences over a year's time (this post #1 at bottom with pics). Many times stronger than oral DMT. Updated 1-1-2022. (this post #1 near middle & bottom)
--> See the 5 hour brightly colored CEV trip report using 300mg oral THH once + 60mg sublingual HPBCD DMT + 35mg sublingual harmine fb x 2 re-doses every 1.5 hour at the very bottom of this post #1. HPBCD DMT kicks ass period. Most meaningful & intense CEV visionary experience of my life, and I've taken Ayahuasca x 70 times and high dose cactus tea over 200 times. Incredible music enhancement the whole time as well. <--
Part 2: L-dreamer's 2/4/2022 experience: "Sublingual DMT + oral THH - surprising and underrated" + Closing tips + Receptorome chart & explanation (post #5)
Part 3: 300mg Tetrahydroharmine (THH) teaching visions all by itself (post #17)
Part 4: Tetrahydroharmine receptorome similarities to mescaline; potentiates cactus & safety note [post #18]
Part 5: Chemist Patrick Arnold's HPBCD complexed prohormones for sublingual use (millions of dollars in sales) & bloodwork studies (post #15)
part 6: Dr. Narang: "with sublingual" or "under the tongue" better than buccal, gingival & palatal, absorption of drugs through the sublingual route is 3 to 10 times greater than oral route and is surpassed by hypodermic injection (post #20)
part 7: a little bit on my 70 Ayahuasca experiences, doses & visions (page 2, post #22)
part 8: New research: Morning glory contains 5 stimulating LSD-like drugs, soluble only in wine/alcohol, only sparingly soluble in water (page 2, post #40)
part 9: 20 minute visionary visit from a dead Aztec Shaman (page 3, post #42)
part 10: One way to make pure tetrahydroharmine (page 3, post #43)
part 11: From the archives of DMT world: How to easily extract 2.3g DMT from 170g bark using a 2 liter Erlenmeyer flask [page 3, post #48]
part 12: Out of print writings on the Divine Plant of the Incas, coca leaf visions...and writings on strong euphoria from coca leaf tea bags soaked in wine, forming orally active cocaethylene in the liver, discovered in 1994. Explains the popularity of Vin Mariani (coca leaf soaked wine) with both popes, Thomas Edison and countless celebrities. (page 3, post #49)
part 13: THH + mushrooms report from JKW (page 3, post #50)
part 14: Tetrahydroharmine + 1-acetaldehyde LSD (similar to ALD-52) combo, like high dose mescaline (page 3, post #56)
part 15: Multiple encounters with death and depression & 80mg DMT complexed to 560mg HPBCD oral Ayahuasca report
[page 3, post #58]
part 16: Late March 2022 experience: Oral Ayahuasca: 250mg oral THH fb + 160mg oral harmine fb + 140mg HPBCD DMT, THH and harmine in gelatin capsules, 140mg HPBCD DMT dissolves from off spoon instantly into 1 shot glass of 125 degree F water, transparent color. Many times more potent than freebase or DMT salts used orally. HPBCD not only helps to mask the taste of the nasty freebase DMT as it goes down but improves absorption into intestinal tissues many factors over. (page 4, post 80)
---------------------------------------------------------------------------------------------------------------------------------------------------
10.18.2021 update, (see page 3, post #56 for details)
Tetrahydroharmine + 1-acetaldehyde LSD (similar to ALD-52) combo, like high dose mescaline:
In closing, I'm going to post what I believe to be a revolutionary psychedelic combination, and it's dirt cheap compared to the rare and very expensive cactus...but it's just as long-lasting, profound, highly euphoric, visual, neon-colorful, music-enhancing & super deep head space, with zero-anxiety as two feet of fat bridgesii.
300mg THH + 250ug 1-acetaldehyde LSD report (2oz fresh cold sherry wine morning glory extract can substitute as well)
1) The combo of 300mg THH + 1-acetaldehyde LSD makes the beauty & aesthetic enhancement way stronger than LSD alone. Same "over the top" beauty enhancement as high dose cactus tea.
2) The music sounds much better than LSD alone, it feels very much like when you combine mescaline with LSD, as THH is like the beta-carboline version of mescaline.
3) The combo is highly visual & neon-colorful with open eyes, with each of the 12 trips spaced two weeks apart experienced so far have seen neon-red-greens, neon-orange-blues, and even neon-purple-yellows, supercolorful just like high-dose cactus tea.
3) Very beautiful combination.
4) This 300mg THH + 250ug 1-acetaldehyde LSD combo is one of my absolute favorites, have since used it every 2 weeks x 12 times now. No re-doses necessary as the THH has a 10.5 hour half life with peak at 5.25 hours. Very powerful: Lasts all evening, infinitely beautiful. I've consistently reached +5 Shulgin level strength every time, very life changing experience every time. Super deep head space, Divine to the extreme, heavenly mescaline-like spiritual euphoria for hours on end, no words to describe.
Note: THH is NOT an MAOI, she (feminine spirit) is a psychedelic SRI or serotonin reuptake inhibitor just like the following psychedelic serotonin reuptake inhibitors: mescaline, LSD, shrooms, ibogaine.
Make sure your THH is pure and not contaminated with unconverted harmaline (which is a RIMA/maoi). Dab some THH on a wet cue tip, rub on paper plate, hold under blacklight, if it glows blue you have THH, if any green glow, you have harmaline in it, keep in mind harmine also glows blue too though.
-------------------------------------------------------------------------------------------------------------------------------------------------
(0) Part 0: 12 reasons pure THH or tetrahydroharmine rocks
-------------------------------------------------------------------------------------------------------------------------------------------------
1. Part 10 of this paper: shows how to convert harmaline to pure THH in 1.5 hour for the first time (very fast) with 75% yield. TIHKAL THH entry also achieved 75% yield. Post also shows how to check the blue glow under blacklight to make sure it is pure. Any green in the glow means you still have un-converted harmaline, but follow instructions and you won't have any unconverted.
1. Dennis Mckenna Ph.D: page 115:
Thus, tetrahydroharmine may prolong the half-life of DMT by blocking it's intraneuronal uptake, and hence, its inactivation by MAO, localized in mitochondria within the neuron.
In my experience, THH doubles the half-life of DMT, so when used sublingually or orally, you get a full strong 90 minutes out of it with long afterglow.
2. She is in the same beta-carboline family as ibogaine. She is the 2nd highest alkaloid in Caapi. She has a 10.5 hour half-life with peak at 5.25 hours.
3. DMT only colors are subdued and dark, but THH brightens the DMT visuals: out of this world impossible bright neon colors are a trait of high dose oral tetrahydroharmine + moderate dose 60 to 70mg+ sublingual or oral HPBCD DMT: neon red-greens, neon orange-blues, neon purple-yellows.
4. DMT does not block serotonin on it's own, but THH does...this results in not only stimulation but euphoria in combo with the DMT: and real Ayahuasca visions become apparent...important teamwork. Ibogaine, LSD, mescaline, shrooms, 5-meo-dmt, bufotenin in Amazonian snuffs, all block serotonin, THH blocks serotonin.
5. THH has numerous similarities to mescaline, she is like the beta-carboline version of mescaline, few people have used her over 100mg. I have seen the receptorome chart for THH vs. mescaline. She not only blocks serotonin like mescaline, but agonizes all 3 adrenal receptors A1-A3 associated with beauty and aesthetic enhancement, just like mescaline. Beauty enhancement is "over the top" when THH is included, she is diamondlike shimmering in her beauty.
Actresses on TV will look like dazzling glowing super-colorful cartoon versions of themselves (just like with high dose cactus tea) only if you include the THH. Researchers have called THH the "tryptamine of the beta-carboline world" and rightly so.
6. THH is found in average 150mg in a cup of Caapi based Ayahuasca tea, when 2 cups are drank by some of the more advanced members for evening at the vegetals (UDV, Santo Daime, Shuar Indian) people are consuming around 300mg of THH.
7. Music will only sound bad-ass incredible if you include from 150mg to 300mg oral THH with your sublingual or oral DMT.
8. This pure THH at 300mg all by herself is extremely visual, she's an isomer of a hormone like substance made in the brain naturally.
9. The entry in TIHKAL for 300mg THH is completely wrong, where the unexperienced person compares it to the effects of 100mg harmaline. She is nothing at all like harmaline, and like 69ron once said about the person's comment in TIHKAL, he or she would not be able to tell their ass from their elbow. I agree, what complete nonsense. Dr. Shulgin wrote that he never got the chance to try THH, but wrote that more studies on it are "badly needed."
10. professor8 (found here from 11/1/2010 he writes like a poet w/special powers of imagination & expression):
Tetrahydroharmine (THH) has the ability to raise your vibration in a most powerful, yet subtle way. It brings a crystalline prismy texture to spice and adds a super clear watery dimension to Aya, like looking down through 10meters of shimmering Caribbean Sea on clear blue day. It brings a dimension of pure light to the entheogenic experience and encourages entities & intelligences of only the Highest Order. If one is not accustomed to perceiving these experiences with a spiritual perspective most of the nuances & subtleties THH brings on are overlooked and remain unseen and one would better enjoy Harmaline as a house painter chooses a roller over a brush, its about preference & choice.
11. Trips (from here on 12/2/2011):
As to how the THH altered the experience -> I find rue extract+DMT to be very similar to mushrooms. I found the THH added to the rue+DMT to shift the experience to a state much closer to that provided by LSD. It was more clear, more energetic, more focused, and when confusion struck it was definitely more "acid-like".
Espiridion:
tetrahydroharmine is much more like mescaline.
The world is moving in the direction of the Left Brain: technology and science. What the world needs is to move in the direction of Right Brain development: empathy, spirituality, connectedness. Compounds like tetrahydroharmine in Caapi could be said to improve emotional intelligence. Is this component of caapi a smart-nutrient for the right side of the brain? you be the judge.
At 300mg of THH all by itself, there are heavy open-eyed tracers like lightening flashes, and hours of teaching closed eye visions that start with colored sparkles and fireworks (red, green, yellow, blue) that dart around and progress into full-fledged way-beyond 4k visions with eyes closed that are not only static but often animated like slow and high speed movies, but all one monochrome color like green or blue for me, when you add DMT, the visions then become colored and patterning on animals for example will display their associated colors, DMT also adds on to or builds on top the THH visions, expanding them, but the teachings and insights & visions are credited to the Vine, just as Gayle Highpine writes in linked paper:
12. Gayle Highpine (Ayahuasca researcher):
The vine carries the content of the message, the teaching, and the insight. The purpose of drinking Ayahuasca is to receive the message the vine imparts.
Tetrahydroharmine or THH ranks very high on the "periodic psychedelic table" among all the known entheogens for inducing realistic way beyond 4k monochrome teaching visions for hours...adding even small amounts of DMT brightens and colorizes the visions, example: reptiles, birds & animals such as serpents/snakes/toucans/parrots/jaguars with patterning show their respective associated colors. Many times I have viewed multi-colored serpents, birds & jaguars several times over hour long CEV periods, serpents are the manifest spirit of Ayahuasca.
Daniel Pinchbeck "Breaking Open the Head" (Daniel also states in his book, that Ayahuasca is his favorite entheogen):
For many people, Ayahuasca-a slowed-down low-res interface of the DMT flash-seems to convey strong messages from the natural world, of nature as sentient energy and spirit matter, of the need to protect the planet we have been given.
Yage whispers that human beings are meant to be gardeners of this reality, journeyers, storytellers and singers, weavers of the sacred. DMT, on the other hand, conveys no overt human or humane message.
Graham Hancock, "Supernatural", pg 428:
My experience with smoked DMT was qualitatively different from the realms and beings Ayahuasca introduced me to. For whereas the Ayahuasca worlds seemed rich, luxurious, and abundant in the transformations of organic and supernatural life, DMT brought me to a world--or to some aspect of a world--that appeared from the outset to be highly artificial, constructed, inorganic, and in essence technological.
Gayle Highpine (Ayahuasca researcher):
In the western world, Ayahuasca acquired a new definition: It was now, by definition, the combination of Banisteriopsis caapi and a DMT-containing plant. Ayahuasca became, by definition “orally active DMT.” The first anthropologist to adopt the new definition seems to have been Luis Eduardo Luna in 1984. Luna spent time with Terence McKenna, absorbing his perspective, before beginning his fieldwork. Since then, anthropologists have increasingly adopted this definition and filtered their observations through it. The preeminence of the Ayahuasca vine in the indigenous Amazonian world became the elephant in the living room of Ayahuasca studies, with a tacit agreement to pretend it doesn’t exist.
The leaves were Ayahuasca’s “helpers,” I was told, and their purpose was to “brighten and clarify” the visions. The vine is like a cave, and the leaf is like a torch you use to see what is inside the cave. The vine is like a book, and the leaf is like the candle you use to read the book.
The vine is like a snowy television set, and the leaf helps to tune in the picture. There was a subtle attitude that the need for strong leaf was the sign of a beginner: An experienced ayahuasquero could see the visions even in low light.
Ayahuasca vine is not visionary in the same way as DMT. Visions from vine-only brewsare shadowy, monochromatic, like silhouettes, or curling smoke, or clouds moving across the night sky. It is because their visions are usually monochromatic that vines are classified by the color of vision they produce: white, black, blue, red (in my experience, dark maroon).
Snakes, the most common vision on Ayahuasca, are considered the manifest spirit of the vine. Vine visions can be hard to see; in fact, the “visions” may not be visual at all, but auditory or somatic or intuitive. But the vine carries the content of the message, the teaching, and the insight.
The leaf helps illuminate the content, but the teachings are credited to the vine. Vine visions are “frequently associated with writing, to a code that is present in visions…or in the ‘books’ where the spirits keep the secrets of the forest.” (Calavia Saez 2011:135).
The vine is The Teacher, The Healer, The Guide. The purpose of drinking Ayahuasca is to receive the message the vine imparts. This is why it is the vine, not the leaf, that is classified by the type of vision it gives. “For them the vine is, in truth, a living guide, a friend, a paternal authority” (Weiskopf 2005:104).
Listening to the Vine:
While I was living in the village, someone began the process of shamanic apprenticeship. There was a series of ceremonies with brews of special strength for that purpose; brews with enormous quantities of vine. About two to three pounds of fresh vine per person was used (about 25 to 35 times the amount needed for MAOI inhibition). Those were powerful experiences indeed.
Although the apprenticeship began with crushingly vine-heavy brews, the more the apprentice progressed, the weaker the brew he would need. He would learn to see the dimmest of visions. If he spent a full two years “fasting,” then eventually even smelling or tasting the brew, even touching an Ayahuasca plant, would be enough to visit her realms. On the other hand, he would learn to navigate the strongest of brews with clear focus, and be undistracted by any amount of DMT fireworks.
Psychedelia, page 61:
---------------------------------------------------------------------------------------------------------------------------------------------------A traditional saying among Ayahuasqueros is that the jungle vine brings powerful realistic visions, but that the chacruna brings light to these visions. According to the view of Western research, this is not the case; essentially the entire psycho-activity resides with the chacruna leaves DMT content.
Ayahuasca researcher Luis Eduardo Luna recently observed that when surveying tribal lore praising the jungle vine, he could find no traces of similar mythology around the two most common plant admixtures; psychotria viridis or diplpterys cabrerana, even though these DMT plants to a Westerner would appear much more important than the harmala alkaloids of the B. caapi liana.
Part 1: HPBCD complexed DMT experimental dosage, effects & duration
---------------------------------------------------------------------------------------------------------------------------------------------------
How this works: the Hydroxy propyl beta cyclodextrin molecule (HPBCD) has an inner hydrophobic cavity (repels water) which attracts & traps freebase molecules like DMT freebase inside it's tornado shaped cone. The outer cavity is hydrophilic (likes water) and thus makes the DMT molecule water-soluble. HPBCD, being a polysaccharide derived from the enzymatic degradation of starch, improves the penetration of the DMT freebase many factors over (studies show x 4 factors or 400 percent into the sublingual mucosa under the tongue.
HPBCD, being a potent polysaccharide derived from the enzymatic degradation of starch, improves the penetration of the DMT freebase many factors over (studies show x 4 factors or 400%) into the sublingual mucosa under the tongue. HPBCD then releases the DMT as the potent freebase into the bloodstream once it crosses the sublingual mucosa, these reasons explain it's potency.
Keep in mind using DMT salts sublingually does not work, and there is no penetration enhancement unless HPBCD is used to complex DMT in the freebase form only. The cyclodextrins have toroidal shapes, with the larger and the smaller openings of the toroid exposing to the solvent secondary and primary hydroxyl groups respectively.
You can order HPBCD from China no matter where you live as it is legal, and if you google "Europe + HPBCD" there are a couple places that sell it as well. It is very common in the USA from *mazon or auction sites.
In closing, complete instructions and pictures all updated for 1-1-2022 on sublingual HPBCD DMT & summary of over 44 sublingual experiences in a year's time (bottom of post #1), these experiences all must stronger than oral DMT by many factors. Zero nausea, zero dizziness, zero anxiety or un-easy feelings, deep head space, highly visual, strong open eyed and CEV's of spinning & dancing geometrics & real Ayahuasca visions, incredible music enhancement, way over the top open eyed beauty, neon colorful & highly euphoric, see trip reports bottom of post #1...will continue to use for the rest of my life, highly recommend: https://www.dmt-nexus.me/forum/default.aspx?g=posts&t=96861
---------------------------------------------------------------------------------------------
Complete instructions and pictures all updated for 1-1-2022 on sublingual HPBCD DMT & summary of over 44 sublingual experiences in a year's time, these experiences all much stronger than oral DMT by many factors. Zero nausea, zero dizziness, zero anxiety or un-easy feelings, deep head space, highly visual, strong open eyed and CEV's of spinning & dancing geometrics & real Ayahuasca visions, incredible music enhancement, way over the top open eyed beauty, neon colorful & highly euphoric, see trip reports bottom of post #1...will continue to use for the rest of my life, highly recommend: https://www.dmt-nexus.me...aspx?g=posts&t=96861
My procedure:
1) place 60mg of DMT onto a spoon
2) add 1:1 molar ratio of host drug to HPBCD powder, this means 1:7 mg ratio DMT to HPBCD, use a 1:8 mg ratio DMT to HPBCD if you are using the 2-Hydroxypropyl-β-cyclodextrin.
3) this means 60mg dmt placed on spoon, then add 420mg of HPBCD on top DMT, use 480mg HPBCD if you are using the 2-Hydroxypropyl-β-cyclodextrin.
4) add 10 drops of very hot near boiling water to the mix from a nearby microwaved coffee mug for DMT doses of 90mg or below, use 12 drops of boiling hot water to mix DMT doses over 90mg (such as 100 to 120mg). 60mg DMT = +3 Shulgin level strength, 90 to 120mg = +5 Shulgin level life changing strength.
5) Knead or crush the HPBCD powder into the dmt using the end of a spoon for 2 minutes, scrape & mix everything back and forth hard using your muscles. This is how scientist pre-pare these complexes by kneading.
6) Optional (you do not have to do this): Hold a lighter far away from under the spoon to heat up spoon for around 20 seconds or so, then pull flame away, this seems to aid dissolution or dissolving after heating up a slight bit, mix the contents a little bit more before using.
7) Take 150 to 300mg tetrahydroharmine orally around 45 minutes before...and take 200mg harmine orally around 45 minutes to 1 hour before...if you don't take harmine orally, then place 25 to 35mg of freebase harmine under your tongue. Then place bottom of your tongue onto HPBCD complexed DMT spoon, the HPBCD DMT glob will all adhere as HPBCD powder forms sticky complexes. Be sure to take the 35mg harmine and HPBCD DMT under tongue all at the exact same time in order to activate the DMT strongly.
Hold under tongue for 10 to 12 or 15 minutes depending on dosage, hold under tongue the whole time to trap sticky liquid complex in the sublingual mucosa. Be sure to use bottom end of tongue to lick any off spoon that is left behind, you want to get it all.
At end of 15 minutes, spit out any saliva into a cup instead of swallowing. Gently relieve any saliva during the 15 minute period as comfort dictates by leaning your head forward and spitting into a cup, but keep your tongue pressed down any time you relieve saliva. There is a mild to moderate sting felt but it's so worth enduring for the effects which begin in 22 minutes or 1/2 the time of an oral dose (44 minutes). The sting does not bother me at all. The tongue is completely fine afterwards (no burn), and it's as if nothing happened the next day.
So long as the HPBCD DMT is used sublingually, there is zero nausea, zero dizziness, no delirium, crystal clear clarity like mescaline...there is something about DMT going thru the stomach that tends to induce nausea and weird uncomfortable strange feelings, just as L-dreamer mentions in post #2 of this thread at the very beginning. Same thing with me. It's also no where near as potent as sublingually used HPBCD DMT. Sublingual HPBCD DMT is at least x 5 times stronger than oral DMT.
---------------------------------------------------------------------------------------------
The combination of THH + DMT simulates true Ayahuasca, but there is zero nausea, zero dizziness, zero queasiness since there is no harmine or DMT going thru stomach and intestines. 22 minutes in there are heavy CEV's of spinning colored geometrics, visions of ancient architecture, animals, aliens, you name it, She seems to tap into the Akashic record of the ether in the Universe, where all past, present, and future is known. Open eyed profound beauty, music sounds incredible.
The sublingual application is several factors stronger than oral DMT, and I've used oral DMT dozens of times. I tolerate this very well compared to oral DMT, extremely pleasurable experience, high spiritual euphoria.
I don't like oral DMT or oral harmine, both make me dizzy, give me anxiety and un-easy feelings, and just keep saying "I want this to end". But the sublingual method is a complete 180 degree, feels great like mescaline all evening long.
Oral 300mg THH taken 45 minutes earlier + sublingual 60 to 120mg HPBCD DMT combined with sublingual 35mg harmine fb journeys (over 44 of them over a year's time). This counts the two subsequent re-doses every 1.5 hour for the evening, for a 4.5 hour total strong journey with super-long afterglow.
First of all there was a deep head space, profound spiritual insights and revelations. I was seeing curtains of visuals in the doorway, closed eye neon spinning & dancing geometrics, music enhancement incredible, out of this world impossible neon colors (like neon yellow-purple) flashing on the walls, some times these colors on the walls would break up into fine lines like lazer beams, and paste themselves like hundreds of beams broadcast on the walls, concentric circle rings in the air, powerful lightening like tracers.
Open eyed beauty so over the top & infinite, actresses in movies looked like glowing, dazzling, super-colorful, cartoon versions of themselves, just like with high dose cactus tea, also zero anxiety just like mescaline. Euphoria was powerful, just like with high dose cactus tea.
Again with the sublingual: pupil dilation maxed out, strong tryptamine body buzz high frequency, heavy CEV imagery, open eyed beauty profound, music sounds incredible.
On my very first 300mg oral THH + sublingual 60mg HPBCD DMT combined with 35mg sublingual harmine fb trip with 2 sublingual re-doses at each 1.5 hour mark (had not used DMT in several months)....all the way till 5am in the morning I was seeing closed eye visions of slow and high speed movies...I saw brightly colored serpents, dungeons I traveled thru, many Mesoamerican pyramids, women of incredible beauty, Japanese landscapes, dancing geometrics, many different animals on a rotating globe, walking on the planet-like globe as it spun, hundreds of visions like slow and high-speed movies over the course of many hours.
I wore headphones and listened to music the whole time, as the music sounded just like if I had taken a very strong cactus tea.
I saw the interiors of many magnificent homes, exposed like a camera flash went off, then off to the next home interior, bizarre alien looking creatures, I saw ancient ruins but they were seen as they were before they fell apart. All sorts of architectural wonders appeared that I could not make out exactly what time period they were from.
All the visions were enchanting & manifested incredible beauty. The multi-colored beautiful serpents kept appearing several times in different forms, as if they have some prominence to do with it all, two of them had shining skin covered in gold scales and intertwined like DNA, reminds me of the Aztec quetzalcoatl myth, the "serpent of precious feathers."
...all of these visions were brightly colored due to the sublingual DMT/harmine and oral THH combo all night long..it was one of the most powerful psychedelic experiences of my life...and I've taken Ayahuasca x 70 times, cactus 200 times, etc...I have never had over 5 hours of non-stop CEV visions anything close to what I saw that first night.
The visions inspired me to buy a book on the Aztec myth of "Quetzalcoatl, the serpent of precious feathers", as I feel somehow this entity is a "teacher to mankind". I saw the brightly colored serpents many times in the 5 hours of visions, and now I understand why they are so commonly reported in Ayahuasca journeys.
They seem to possess divine knowledge that humans were not supposed to have been privileged to, but the serpents gifted this knowledge to humankind.
Recently found a 1.5 hour video on Amazon prime entitled "Ancient Alien Origins" which is all about this ancient alien flying serpent or dragon entity which is found in all religions of the world & "BAM, Builders of the Ancient Mysteries".
Return of the Serpent & of Eden:

Return of the Serpent & of Eden
We have been called “witches”, “sorceresses”, and the patriarchal lines would say that we were the downfall of man (i.e. the story of the garden of Eden). The global conscio…
https://en.wikipedia.org/wiki/Mind_at_Large
300mg of tetrahydroharmine (THH) is equated to the (CEV) power of 100mg harmaline, but without all the nausea and dizziness. It glows blue under blacklight, like LSD or psilocin & has a metallic-like lingering taste with a 10.5 hour half-life.
"Cyclodextrins used as excipients" pdf, European Medicines Agency, Oct 9, 2017: page 5 of 16 under "Oral products, Kinetics":
The oral bioavailability of cyclodextrins is very low in adult animals and humans (0.1–3 percent except for RM-β-CD, which has a bioavailability of 12% in rats. Because of their bulky and hydrophilic nature only insignificant amounts of cyclodextrins are absorbed from the gastrointestinal tract by passive diffusion [33, 27].
This pharmacology has been used to potentiate these freebase drugs ORALLY as well -- in my experience, this results in an Ayahuasca experience that is strong in potency. Example: HPBCD improves oral absorption profile for Ofloxacin, a second generation fluroquinolones by 54 to 89 percent.
I've used HPBCD complexed DMT orally three times stirred into a 1oz hot water tea (it dissolves instantly) with 150 to 300mg THH + 160 to 200mg harmine with a bit of citric acid to help the harmalas dissolve, and it's also way more potent than normal DMT freebase or salts used orally, very reminiscent of my over 70 Hawaiian psychotria brewed leaf teas, very strong & all encompassing. Trip report on page 2, post #25.
But I vastly prefer the oral 150 to 300mg THH + sublingual Ayahuasca (35mg sublingual harmine fb + 60 to 100mg sublingual HPBCD DMT all taken at exact same time under tongue), many times stronger than oral DMT, and zero dizziness or un-easy feelings for me due to only 35mg harmine being used. Highly recommend for sensitive individuals like myself. Very strong & feels great all evening like mescaline, heavenly.
"Sublingual mucosa as a route for systemic drug delivery" by Narang & Sharma 2010:
https://innovareacad...Suppl2/1092.pdf
As you can see from this sublingual viagra study, even 50 to 100mg doses can be administered under the tongue, the authors noting that less of the drug was required, and that it began working in only one half the normal time of an oral dose:
"The start of pharmacological activity after sublingual administration of sildenafil citrate in 30 patients affected by erectile dysfunction." by Siati & Franzolin 2003:
https://pubmed.ncbi....h.gov/12741340/
Tetrahydroharmine on it's own will also yield the same type visions as harmaline, it just takes more of it. For example, around 300mg of THH will yield the same visions as about 100mg harmaline...even if the THH dose is split in two over several hours, the visions will still be apparent some time after the 2nd dose takes effect, the doses are additive.
THH in the caapi also seems to strongly activate the right hand hemisphere of the brain-- the side that performs tasks that have do with creativity and the arts, feelings, visualizations, imagination, holistic thinking & intuition, empathy, spirituality & connectedness. Researchers found that the right side of the brain lit up in brain scans of people who took LSD, mescaline, or mushrooms. This includes tetrahydroharmine. The world is largely moving in the direction of the Left Brain: technology and science. What the world needs is to move in the direction of Right Brain development.
Quote from TIHKAL by Dr. Shulgin "More studies on tetrahydroharmine are absolutely imperative."
References:
Dennis Mckenna Ph.D:
I have years of experience with pure tetrahydroharmine, and it does indeed do this very well.Thus, tetrahydroharmine may prolong the half-life of DMT by blocking it's intraneuronal uptake, and hence, its inactivation by MAO, localized in mitochondria within the neuron.
Jamie, posted : 11/23/2012 8:29:28 PM:
I use 35mg freebase harmine sublingually along with the sublingual HPBCD complexed DMT, 35mg sublingual has the power of x6 or 210mg oral harmine, but without the dizziness and un-easy feelings you often feel with an oral dose.You can't compare sublingual or oral either..20-30mg sublingual harmine is enough to activate sublingual DMT and cause effects on it's own. 20mg sublingual is probably comparable to 200mg oral.
Dr. Narang:
In over 44 total sublingual experiences over a year's time, this sublingual route is many times more potent than oral DMT. The only way I use it.The absorption of drugs through the sublingual route is 3 to 10 times greater than oral route and is surpassed by hypodermic injection. Sublingual mucosa under tongue is only 100 to 200 microns thick.
"Cyclodextrins used as excipients" pdf, European Medicines Agency, Oct 9, 2017:, under "Kinetics":
HPBCD enhances the drug permeability of guest complexed drug, but the cyclodextrins themselves are poorly absorbed via the sublingual mucosa.Cyclodextrins at high doses can increase drug permeability by direct action on mucosal membranes and enhance drug absorption and/or bioavailability. These effects are possibly caused by solubilisation of membrane lipids through inclusion complexation with cyclodextrins and the ability of cyclodextrins to cause perturbation of membrane integrity. However, unlike detergents, cyclodextrins solubilize membrane components without entering into the membrane, therefore the perturbing effects of cyclodextrins are mild and reversible [7].Cyclodextrins are absorbed poorly via mucosal membranes.
Pic 1: How to mix the DMT, HPBCD, and drops of very hot water on a spoon.
Pic 2: 1kg container of HPBCD from China for cheap. 2-hydroxy-propyl-beta-cyclodextrin is the more common form available, works the exact same.
Pic 3: Sublingual mucosa under tongue only 100 to 200 microns thick.
Pic 4: 300mg pure THH for oral use in weigher, 35mg harmine fb for sublingual use next to it, 60mg dmt fb in spoon, 420mg HPBCD white powder above it, coffee mug of near boiling hot water, medicine dropper pipette, Bic lighter.
Pic 5: 60mg DMT was covered with x 7 or 420mg HPBCD powder and 10 drops of near boiling hot water from a nearby coffee mug was added, and all mashed and stirred well for 2 minutes, then Bic lighter held under spoon until the solution just started to boil (20 seconds or so), then pull flame away, notice how well the DMT complexed inside the HPBCD.
Take 150 to 300mg tetrahydroharmine 45 minutes earlier orally, place 35mg harmine under tongue, then place tongue on to HPBCD complexed DMT spoon, it will all adhere for sublingual use. Journey starts in 22 minutes after application and lasts x 90 minutes, at which time you can re-dose x 2 more times, each dose just as strong as 1st dose for a 4.5 hour long strong journey with super-long afterglow. Instructions, 5 hour CEV trip report and 9 pics here on post #1.
--------------------------------------------------------------------
Stay true to yourself, Love, Peace and Music
http://www.friskyradio.com




 Sublingual mucosa as a route for systemic drug delivery (2).pdf 240.7KB 3 downloads
Sublingual mucosa as a route for systemic drug delivery (2).pdf 240.7KB 3 downloads Thermodynamic properties of hydroxypropyl Beta cyclodextrin and guest interaction, a survey of recent studies, 2021.pdf 854.1KB 6 downloads
Thermodynamic properties of hydroxypropyl Beta cyclodextrin and guest interaction, a survey of recent studies, 2021.pdf 854.1KB 6 downloadsquestions-answers-cyclodextrins-used-excipients-medicinal-products-human-use_en.pdf 273.35KB 5 downloads
 HPBCD solubility enhancement table for 68 drugs.pdf 61.76KB 5 downloads
HPBCD solubility enhancement table for 68 drugs.pdf 61.76KB 5 downloads
Last edited: