Okay, let me try to explain benzodiazepines as simple and quickly as possible.
Take a look at the
Wiki page, especially the structure to the right, that's the benzodiazepine core found in all benzodiazepines. There are multiple positions that can be substituted with different substance groups.
R7 is always a halogen (bromine or chlorine) or a nitro group. Bromine is most potent, but also most hypnotic; nitro is most recreational.
R2' can be nothing or a halogen (chlorine, fluorine). Halogens tend to increase the potency a lot.
R2 is pretty much always a ketone (except for triazolobenzodiazepines, which I'll explain below).
R1 can be nothing or a methyl group (again, exception triazolobenzodiazepines).
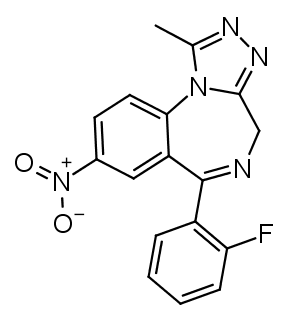
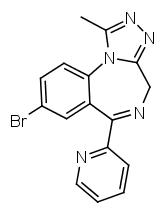
In triazolobenzodiazepines (such as
Alprazolam) R1 and R2 are infused into a 1,2,4-triazole ring. The methyl group on the triazole ring can be removed, this roughly halves the potency, but increases the duration (see Estazolam or Metizolam).
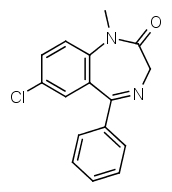
Let's take a look at diazepam:
R7 is a chlorine group, R1 methyl.
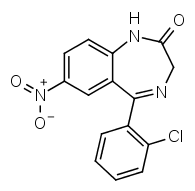
Compare that to clonazepam:
R7 is a nitro group, R2' chlorine. The halogen at R2' gives it a much higher potency compared to diazepam.
Triazolobenzodiazepines with a R7 nitro group tend to be the most euphoric/recreational benzodiazepines.
The name Flu-ni-trazolam tells you all about the structure already. Flu = fluorine at R2', ni = nitro at R7, triazolam = triazolo group. Similar how Clonazepam tells you that there's a chlorine group at R2' and a nitro one at R7.
Flunitrazolam combines the most recreational substitutions (triazolo and R7 nitro) with R2' fluorine, which provides the highest potency.
Of course there're also a few more possible changes, such as 3-methylbenzodiazepines (which are metabolites of other benzodiazepines), for example oxazepam, which is a metabolite of diazepam.
It is also possible to replace the phenyl ring with a pyridine one, as seen in pyrazolam. This seems to be of similar potency as a 2-chlorophenyl group, but causes the substance to be active at different subreceptors.
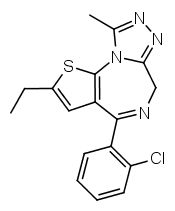
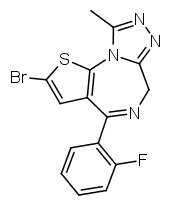
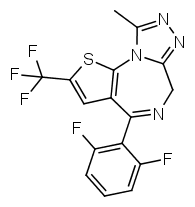
Another possibility is going from benzodiazepines to thienodiazepines. Here the upper phenyl ring is replaced by thiophene. This also increases the potency a bit. An example is etizolam.
The most potent possible benzodiazepine derivative would therefore be something like Flubrotizolam (Flu = R2' fluorine, bro = R7 bromine, ti = thiophene, zolam = triazolo).
Does that make any sense to you? If not, PM me with any questions you have instead of going completely offtopic here