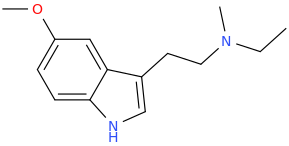
Heh. We would have got on in school, sekio.
We'd have got on like CS2, WP, and donald trump's political manifesto. You'd probably have been the only other person in the class not to duck under the table after that potassium lump in the fish tank...would you believe me if I said it was an accident? (if so, I've got a mansion in nigeria I'd be happy to sell you for a few hundred quid, only, I'd need you to transfer 100 K, which would be refunded, into my bank account due to nigerian tax laws needing a foreign citizen to provide references and financial security

)
(No, there were no fish in there, I'm wasn't THAT kind of kid, the kind of sick little fuck who'd pull the wings off flies, or microwave kittens. There are plenty of things I WOULD do with a microwave at that age that most people wouldn't think of in a thousand years, and of those that would, quite a lot of them wouldn't try it if you paid them, and most of them destructive in an amusing sort of way, none of them however, included aiming at living things other than plant life) It was just a convenient tank to be used for the demonstration of what a fair sized chunk of 'sodium metal' does when it goes into water. And cut to according proportions for the element it was believed and intended to be by the rest of the class, staff included. Ever seen a big lump of potassium go into a tank of water on top of a high bench in a room with a low ceiling? the way it dribbles down in a purple-flaming waterfall of molten metal, right back down into the water when it starts dripping back down off the ceiling, it's quite spectacular. Up, back down, back up again, back down, back up....like a chemistry nerd kid's version of a slinky that no responsible parent would dream of buying their kid for their birthday/xmas/any other reason that didn't involve a demand at gunpoint

)
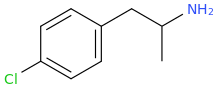
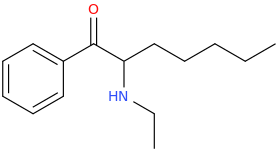
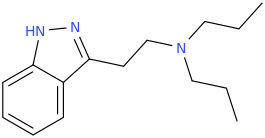





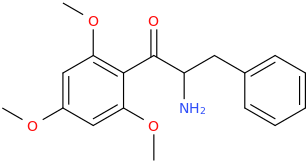
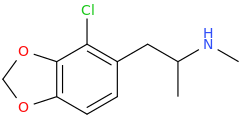
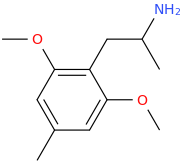
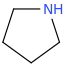
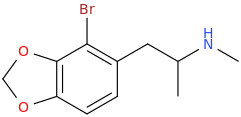
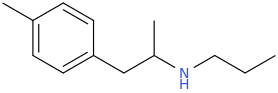
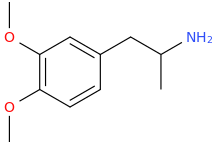
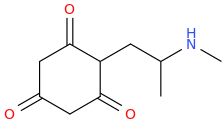
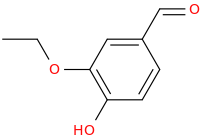
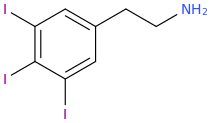
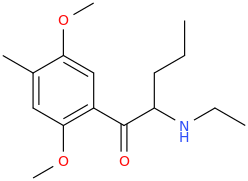
 )
)